A Close Look at the Dynamics of an Ion–Neutral Reaction
Reactions between ions and neutral atoms or molecules occur in various settings, from planetary atmospheres to plasmas. They are also the driving force behind rich reaction chains at play in the interstellar medium (ISM)—the giant clouds of gas and dust occupying the space between stars. The ISM is cold, highly dilute, and abundant with ionizing radiation [1]. These conditions are usually unfavorable for chemistry. Yet, more than 300 molecular species have been detected in the ISM to date, of which about 80% contain carbon [2]. Now Florian Grussie at the Max Planck Institute for Nuclear Physics (MPIK) in Germany and collaborators report an experimental and theoretical study of an ion–neutral reaction: that between a neutral carbon atom and a molecular ion (HD+), made of a hydrogen and a deuterium (heavy hydrogen) atom [3, 4]. The study’s findings could improve our understanding of the chemistry of the ISM.
Ion–neutral reactions are fundamentally different from those involving only neutral species. Unlike typical neutral–neutral reactions, ion–neutral reactions often do not need to overcome an activation energy barrier and proceed efficiently even if the temperature approaches absolute zero. The reason for this difference is that, in ion–neutral reactions, the ion strongly polarizes the neutral atom or molecule, causing attractive long-range interactions that bring the reactants together.
Ion–neutral reactions have been extensively studied since the 1960s [5]. In many ion–neutral experiments, the ions are created by bombarding a neutral gas with fast electrons. However, this process causes the generated ions to be in highly excited vibrational and rotational states. By instead preparing the ions in specific low-energy states, comparisons between theory and experiment can be greatly simplified.
To achieve that feat, Grussie and collaborators turned to an impressive experiment built at the MPIK [6]. This setup can replicate the ultralow pressures and temperatures of the ISM and can be used to carefully study ion–neutral reactions. A key component of the experiment is the Cryogenic Storage Ring—the largest machine of its type, with a circumference of about 35 m. The researchers used this ring to store HD+ molecular ions for up to 20 seconds, which gave the ions enough time to relax to their ground vibrational state and low-energy rotational states.
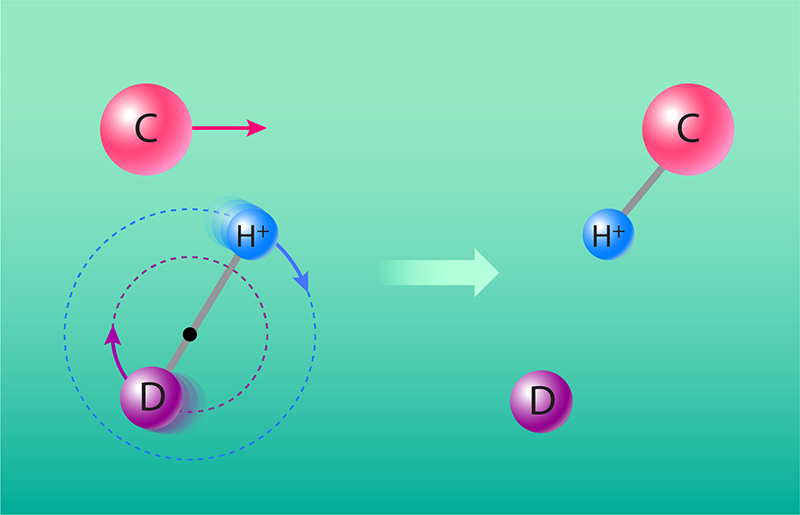
Grussie and collaborators studied the HD+ + C reaction by injecting carbon atoms into a section of the ring with the stored HD+ ions. The researchers were able to control the speed of the carbon atoms by initially giving them an extra electron. These negatively charged carbon ions could be easily accelerated and decelerated by static electric fields, before being neutralized by laser light. The speed-tuned carbon atoms then entered the ring tangentially, so that they spatially overlapped the HD+ ions. Collisions were studied at energies between about 60 meV and 10 eV, determined by the relative velocity of the two reacting species.
The researchers were able to measure the reaction’s absolute rate coefficient—a fundamental parameter that quantifies a reaction’s speed. This task is, in general, notoriously difficult to perform for reactions in the gas phase, such as this one. Compared with experiments involving rotationally and vibrationally excited ions [7], the measured rate coefficient reported by Grussie and collaborators is much closer to that predicted by standard reaction models.
Additionally, the researchers found that the reaction more often produced CH+ + D than CD+ + H (Fig. 1). In other words, it was more likely for the carbon atom to extract an H+ ion (rather than a D+ ion) from the HD+ molecular ion—an example of the so-called intramolecular kinetic isotope effect. In typical neutral–neutral reactions with an activation energy barrier, this effect is usually explained by the difference in the zero-point energies (the lowest possible vibrational energies) of the hydrogen- and deuterium-containing species. However, the HD+ + C reaction is exothermic and barrierless, and the difference in the zero-point energies of CH+ and CD+ ions is too small—relative to the energy released in the reaction—for it to significantly affect the reactivity. Instead, the researchers argue that the observed kinetic isotope effect arises from the displacement of the HD+ ion’s center of mass toward the deuterium atom. They suggest that this displacement leads to an enhanced rate coefficient for the formation of the lighter, CH+ molecular ion [8, 9].
Next, by theoretically studying the short-range interactions of an HD+ ion and a carbon atom, Grussie and collaborators were able to estimate the probability that a close encounter of these species would result in a reaction—a missing ingredient in standard reaction models. These calculations fully explain the obtained experimental data over the full range of energies studied. Additionally, by considering the effect of the electric quadrupole moment of the carbon atom on the long-range interactions between the reactants, and combining this insight with the estimated reaction probability, the researchers were able to correctly predict the measured rate coefficients at the lowest energies studied.
The implications of this work extend beyond the specific reaction and range of collision energies considered. A main takeaway is that, for exothermic ion–neutral reactions relevant to atmospheric and interstellar chemistry, it is often possible to use relatively simple kinetic models to predict the differences in the rate coefficients for different isotopes. Moreover, the reported preference for the formation of the lighter molecular ion in such a reaction seems contrary to the intriguing observation that deuterium-bearing species can be much more abundant in the ISM than expected [10]. Finally, it is now clear that the ion’s vibrational state, the short-range interactions, and the effect of multipole moments on the long-range interactions all need to be fully considered to obtain reliable rate coefficients and product yield ratios to use in models of reaction chains in the ISM.
References
- T. P. Snow and V. M. Bierbaum, “Ion chemistry in the interstellar medium,” Annu. Rev. Anal. Chem. 1, 229 (2008).
- B. A. McGuire, “2021 census of interstellar, circumstellar, extragalactic, protoplanetary disk, and exoplanetary molecules,” Astrophys. J., Suppl. Ser. 259, 30 (2022); Cologne Database for Molecular Spectroscopy, Molecules in space, https://cdms.astro.uni-koeln.de/classic/molecules.
- F. Grussie et al., “Merged-beams study of the reaction of cold HD+ with C atoms reveals a pronounced intramolecular kinetic isotope effect,” Phys. Rev. Lett. 132, 243001 (2024).
- F. Grussie et al., “Absolute rate coefficient measurements of the reactions of vibrationally cold HD+ and H+3 ions with neutral C atoms,” Phys. Rev. A 109, 062804 (2024).
- V. G. Anicich and W. T. Huntress, Jr., “A survey of bimolecular ion-molecule reactions for use in modeling the chemistry of planetary atmospheres, cometary comae, and interstellar clouds,” Astrophys. J., Suppl. Ser. 62, 553 (1986).
- R. von Hahn et al., “The cryogenic storage ring CSR,” Rev. Sci. Instrum. 87, 063115 (2016); F. Grussie et al., “An ion-atom merged beams setup at the Cryogenic Storage Ring,” Revi. Sci. Instrum. 93, 053305 (2022).
- P.-M. Hillenbrand et al., “Experimental study of the proton-transfer reaction C + H2+ → CH+ + H and its isotopic variant (D2+),” Phys. Chem. Chem. Phys. 22, 27364 (2020).
- J. C. Light and S. Chan, “Isotopic distributions in exothermic ion–molecule reactions. A simple model,” J. Chem. Phys. 51, 1008 (1969).
- A. Henglein, “Stripping effects in ion-molecule reactions,” Ion-Molecule Reactions in the Gas Phase, edited by P. J. Ausloos (American Chemical Society, Washington, DC, 1966), p. 63.
- T. J. Millar, “Deuterium in interstellar clouds,” Astron. Geophys. 46, 2.29 (2005).