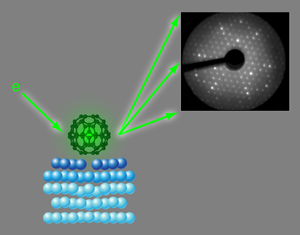
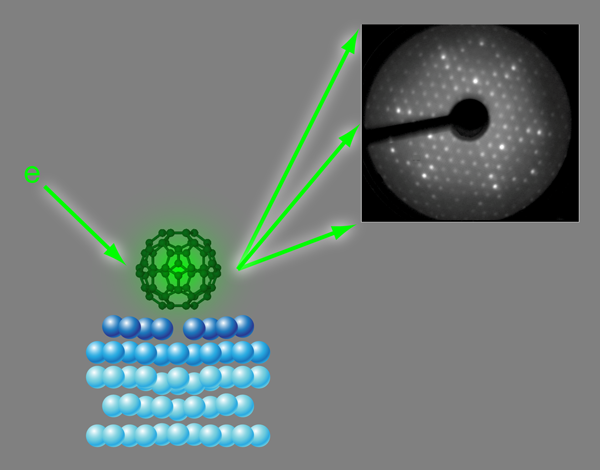
Nanospheres on a silver plate
Ever since the 1985 discovery of buckminsterfullerene (formally known as C60), this molecule, with its perfect shape and high symmetry, has fascinated scientists, physicists, and chemists alike [1–3]. Similar to a soccer ball, the molecule consists of 20 hexagons and 12 pentagons, with carbon atoms at the vertices (Fig. 1). The carbons are connected through alternating single and double bonds, similar to the bonding in graphite or graphene (a single graphite layer). As a result of its high symmetry and conjugated bond structure, the electronic properties of C60 are very unusual, and there is a massive research effort toward integrating it into molecular scale electronic devices [4].
In this context, it is important to understand how the molecule forms bonds with a metal substrate, such as silver, which is commonly used as an electrode material. The local arrangement of silver and carbon atoms at such an interface will affect the strength and stability of the metal–molecule bond as well as the electronic structure of the C60 molecule itself. Silver is commonly considered a relatively unreactive metal that only forms strong bonds with very electronegative atoms such as oxygen, sulfur, or halogens. The silver d electrons are too low in energy to have significant overlap with the frontier orbitals of most organic molecules, and organic molecules usually do not form strong adsorption bonds with silver surfaces.
On the close-packed {111} surface of silver, C60 molecules form an ordered structure, which has been studied with scanning tunneling microscopy (STM) or x-ray photoelectron diffraction as well as theoretical model calculations. So far, the results have been conflicting. Neither theory nor experiment have been able to reveal, unambiguously, which part of the molecule (the hexagonal or pentagonal ring) faced the surface or the site where the molecule was adsorbed (right on top of a silver atom or in the hollow site between three atoms). Now, in a paper appearing in Physical Review Letters, Hsin-I Li, Renee Diehl, and colleagues at Penn State University, University Park, Pennsylvania, US, joined by an international group of scientists from the US, Finland, Germany, and the UK, have determined the geometry of C60 on the Ag{111} surface using a technique called low-energy electron diffraction (LEED) [5].
LEED is an experimental diffraction technique, in principle similar to x-ray diffraction: electrons with kinetic energies between about 50 and a few hundred electron volts ( eV) have wavelengths around 1Å [λ=(150eV/E)1/2 Å]. This is the same length scale as the wavelengths of x rays and interatomic distances in molecules and most solids. Therefore diffraction patterns, similar to x-ray Laue patterns, are observed if a collimated beam of low-energy electrons impinges on ordered structures of atoms or molecules (Fig. 1, Upper right). Unlike x rays, however, electrons in this energy range interact very strongly with atoms in their path and penetrate only a few nanometers of solid matter. Because adsorbed atoms or molecules typically only affect the first few atomic layers of a surface low-energy electron diffraction is an ideal probe for the crystallography of single crystal surfaces.
In the LEED experiment the intensities of diffraction spots are recorded as a function of electron energy (or, equivalently, wavelength). The geometry of the atoms at the surface is determined by comparing these intensity-versus-energy curves with calculated curves for guessed geometries [6,7]. These model geometries are optimized until good agreement is achieved between experimental and calculated data. This strategy is similar to structure determination in x-ray crystallography; however, the strong interaction between electrons and solids makes the model calculations much more demanding in terms of computer power. So far, with LEED it has been possible to determine the adsorption geometries of relatively small molecules, typically up to the size of benzene. The work by Li et al. in which they are solving the structure of a surface covered with molecules, each consisting of 60 atoms, therefore represents a major step forward in terms of the structural complexity that can be determined by LEED.
At first sight, the structure that Li et al. find is somewhat surprising. The bonds between the carbon atoms in C60 and the silver surface atoms are normally thought to be predominantly ionic and weak in comparison with the metallic silver–silver bonds within the substrate surface and the covalent bonds within the C60 molecule, which is one of the most stable configurations of carbon atoms. Thus, one might expect that the adsorption geometry is essentially determined by which arrangement optimizes the close packing of “molecular soccer balls” and that both the molecule and the silver surface are not much different in contact with one another than they are apart. The analysis of the diffraction measurements shows, however, that this is not the case. The molecules stay essentially unchanged but are able to “dig” nanosized holes into the metal surface by removing the silver atom underneath their adsorption site (Fig. 1). The positions of the other substrate atoms change very little. No indication of such “vacancy reconstruction” was found in any of the previous experiments mentioned above, since they only probed the properties of the molecules and not the geometry of the underlying substrate.
In order to explain this result, the group also used density-functional theory to calculate the adsorption energies for this vacancy structure and a number of other possible geometries without such a reconstruction. These calculations reveal that the vacancy structure is indeed the one with the strongest surface bond, even when the cost of creating the vacancy, 0.67eV, is taken into account. It appears that the six lower-coordinated silver atoms surrounding the vacancy site are able to form much stronger bonds with the molecule (there is a carbon atom pointing towards each of them) than the silver atoms surrounded by the maximum number of neighbors in the {111} surface. This anticorrelation between bond strength and coordination is a well-known phenomenon in molecular adsorption on metal surfaces and clusters [8].
The fact that there is an activation barrier associated with creating these vacancies explains why earlier STM and LEED studies found that the adsorbed C60 molecules were more ordered after annealing the substrate at relatively high temperatures (around 600K). At these temperatures, the silver atoms are sufficiently mobile that they can rearrange to accommodate the vacancy structure.
In their paper, Li et al. also predict similar C60-induced vacancy structures on close-packed surfaces of gold and aluminum. An earlier experimental surface x-ray diffraction study for C60 on Pt{111} came to the same conclusion [9], though with less detail of the surface geometry. The general trend in all of these cases shows that even molecules with relatively weak individual (atom-to-atom) surface bonds can induce substantial substrate reconstructions in order to create favorable adsorption sites [8]. Such “nanopatterning” of substrates is essential to the stability of ordered structures of these molecules and can critically influence their electronic structure, which is an important aspect in the design of molecular electronic devices. Since these effects take place in the layers below the molecular surface layer, they cannot be detected easily through microscopic techniques such as scanning tunneling microscopy, but diffraction techniques using x rays or low-energy electrons also probe the atomic layers underneath the surface and can reveal their three-dimensional structure. Li et al. have proved that this can be done for molecules with up to 60 atoms and have thus opened the door to studies of a large number of technologically and biologically important interface structures.
References
- H. W. Kroto, J. R. Heath, S. C. O’Brien, R. F. Curl, and R. Smalley, Nature 318, 162 (1985)
- R. F. Curl and R. E. Smalley, Science 242, 1017 (1988)
- H. W. Kroto, Angew. Chem. 31, 111 (1992)
- Perspectives in Fullerene Nanotechnology, edited by E. Ōsawa (Kluwer Academic, New York, 2002)[Amazon][WorldCat]
- H. I. Li, K. Pussi, K. J. Hanna, L-L. Wang, D. D. Johnson, H-P. Cheng, H. Shin, S. Curtarolo, W. Moritz, J. A. Smerdon, R. McGrath, and R. D. Diehl, Phys. Rev. Lett. 103, 056101 (2009)
- J. B. Pendry, Low Energy Electron Diffraction: The Theory and Its Application to Determination of Surface Structure (Academic, London, 1974)[Amazon][WorldCat]
- M. A. Van Hove, W. H. Weinberg, and C.-M. Chan, Low-Energy Electron Diffraction: Experiment, Theory, and Surface Structure Determination, Springer Series in Surface Sciences Vol. 6 (Springer, Berlin, 1986)[Amazon][WorldCat]
- G. A. Somorjai, Chemistry in Two Dimensions: Surfaces (Cornell University Press, Ithaca, NY, 1981)[Amazon][WorldCat]
- R. Felici, M. Pedio, F. Borgatti, S. Iannotta, M. Capozi, G. Ciullo, and A. Stierle, Nature Mater. 4, 688 (2005)