Carbon Dating with Lasers
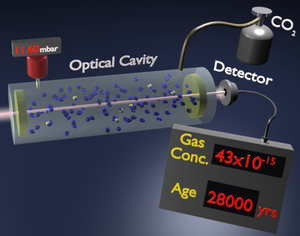
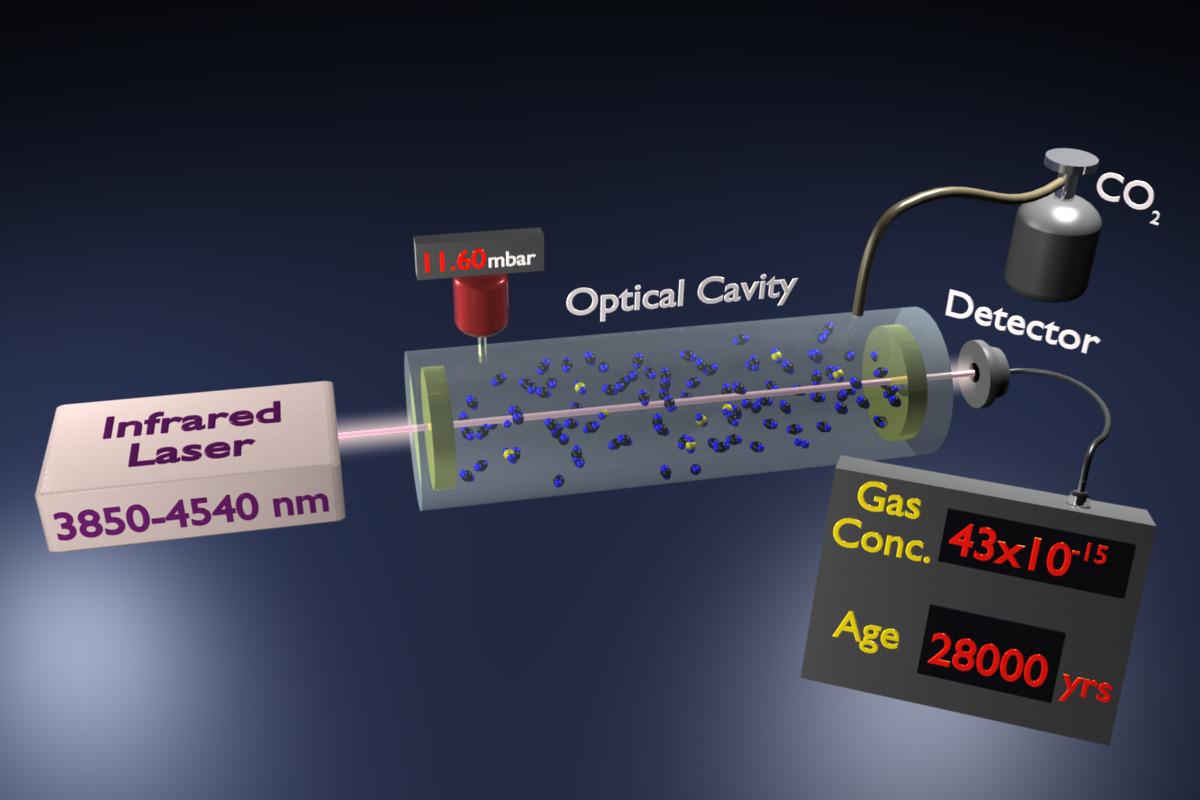
A new optics-based method for detecting trace gases could offer a potential alternative way to date archeological artifacts. As described in Physical Review Letters, the technique involves using infrared laser light to detect tiny amounts of carbon- 14 in a gas sample. The system can detect a trace gas at a pressure of half of a femtobar, 10-15 times atmospheric pressure, a lower pressure than any previous method has detected for a simple molecular gas. The sensitivity still needs to be improved to compete with state-of-the-art carbon dating techniques, but the new method has a relatively small size and cost. It could also be practical in fields such as pharmaceutical testing and environmental monitoring.
Carbon dating relies on carbon- 14, a radioactive isotope with a half-life of 5730 years. Living things have about one carbon- 14 atom per trillion carbon- 12 atoms, which reflects the relative abundance in the atmosphere. But once an organism dies, it no longer exchanges carbon with the environment. The carbon- 14 in an old piece of paper or clothing, for example, continuously decays, and the amount remaining reveals the object’s age.
Archaeologists typically measure the remaining carbon- 14 using accelerator mass spectrometry (AMS), in which part of the sample is burned to make carbon dioxide, and then ions extracted from this gas are sorted by mass. The technique can measure a carbon- 14 to carbon- 12 ratio down to one part per quadrillion ( 10-15), which corresponds to a sample age of 50,000 years.
Another highly sensitive technique is optical spectroscopy, which detects small quantities of a substance by measuring the amount of light it absorbs. The range of wavelengths between 2.5 and 10microns is where gas molecules typically have their strongest absorption, but the technology for high-precision measurements in this region has only recently become available.
Paolo De Natale of the Italian National Research Council and the European Laboratory for Non-Linear Spectroscopy (LENS), both in Florence, Italy, and his colleagues, unveiled a new high-sensitivity technique last year called saturated-absorption cavity ring-down spectroscopy (SCAR) . In conventional cavity ring-down spectroscopy, which has been around for over 20 years, researchers fill a cylindrical cavity with a gas sample and briefly shine light into the cavity at a wavelength where the trace gas absorbs. After turning off the light, mirrors at each end continue to reflect the photons back and forth thousands of times until all of the light goes away.
The time it takes for this so-called “cavity ring-down” depends on the degree to which the mirrors are imperfect reflectors but also on the amount of light absorbed by the trace gas. To distinguish the two effects, researchers ordinarily measure the reflection losses separately with an empty cavity. But the system De Natale and his colleagues have developed is 20 times more sensitive because it can isolate the two types of losses with the sample present. For this calibration step, the system essentially turns off the absorption for a short time by using a laser with sufficient power to excite all of the target molecules, putting them into a state in which they can’t absorb light. After about 10microseconds, absorption returns as the molecules fall back into their ground state.
The team has now pushed the sensitivity of the system to its limits by targeting rare carbon dioxide molecules containing carbon- 14. By comparing carbon dioxide samples with natural and depleted abundances of carbon- 14, the team showed that their method could detect carbon- 14 at the level of 43 parts per quadrillion, which corresponds to a sample age of 28,000 years. Although not yet as sensitive as AMS, the SCAR system fits on a table top and costs ten times less than an AMS machine, says coauthor Giovanni Giusfredi. There is also a wide variety of radiocarbon applications that don’t require such high sensitivity and that might benefit from 3SCAR’s compact size, such as studying the carbon cycle in different geographic regions and evaluating drug metabolism.
“I think this represents a solid advance in spectroscopic sensitivity,” says Barry McManus from Aerodyne Research, Inc., in Billerica, Massachusetts. “The authors show three times better detection limits than any yet reported for an absorption spectroscopy experiment.” Gianfranco Di Lonardo from the University of Bologna in Italy believes this new technique will improve with further development to become more competitive with AMS-based carbon dating.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- G. Giusfredi, S. Bartalini, S. Borri, P. Cancio, I. Galli, D. Mazzotti, and P. De Natale, “Saturated-Absorption Cavity Ring-Down Spectroscopy,” Phys. Rev. Lett. 104, 110801 (2010)
More Information
What is AMS? (from Prime Lab at Purdue University)
Cavity Ring-Down Spectroscopy by Giel Berden