New Vibration in Material That Shrinks When Heated
Heating normally makes solids expand, but some materials shrink when heated. One of these has now exhibited a previously unobserved crystal lattice vibration, as reported in Physical Review Letters. The “atomic springs” that control the vibration become stiffer the more they stretch, unlike the usual classical spring case, where the stiffness is constant. This “quartic oscillation” represents an unexplored realm of the physics of crystals that may play a part in other materials and could lead to the development of materials with novel thermal properties. The new analysis also suggests that revisions may be needed for previous theories of the shrinking-on-heating behavior.
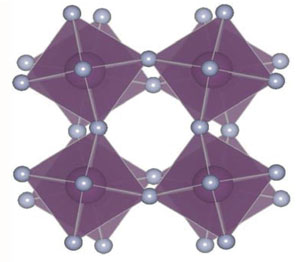
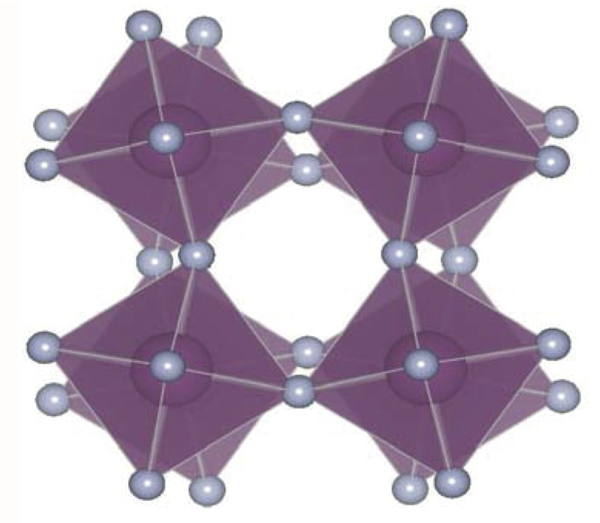
Materials with negative thermal expansion (NTE)—or heat-shrinkage—could be combined with conventional materials to produce tooth fillings, telescope mirrors, and other structures for which a fixed shape and size are desirable over a wide range of temperature. The poster child for NTE is zirconium tungstate ( ), which exhibits a large thermal contraction of up to 0.001 percent per degree Kelvin over a range of a thousand degrees. Geometric models of zirconium tungstate assume that when heated, the tetrahedra and octahedra in the atomic structure remain rigid but can pivot or rock relative to each other to reduce the total volume, the way an accordion folds (see animations from 22 November 2004 Focus story).
Models of these so-called rigid unit modes, which also occur in related materials, do not typically consider the forces that control the rocking motion, says Brent Fultz of the California Institute of Technology (Caltech) in Pasadena. He says it’s not easy to analyze the forces inside a complex crystal like zirconium tungstate, whose unit cell—the smallest repeating structure in the crystal—consists of atoms. But last year researchers discovered that a simpler compound, scandium trifluoride ( ), has NTE comparable to zirconium tungstate over a temperature range from to [1]. This material has a unit cell of just atoms, making it easier to analyze.
To probe the forces acting inside scandium trifluoride, Fultz and his colleagues began by using neutron scattering to measure the vibrational spectrum—essentially the resonant frequencies of the various modes of vibration for the atoms, which act like masses connected by springs. They measured at temperatures between and , and as expected, most of these resonances remained roughly constant as the temperature varied. However, one of the resonant features surprisingly shifted to higher frequency, as if the springs were stiffening as temperature increased.
To understand this stiffening, the researchers performed calculations that were the equivalent of vibrating the crystal structure at a single frequency and then watching how the atoms responded. In most cases, vibrating atoms experience a “springlike” restoring force that is proportional to the amount of stretching, which implies a potential energy well that increases as the square of the separation between atoms. However, some of the modes in scandium trifluoride were clearly vibrating in a quartic potential, which increases as the fourth power of the separation.
Because the quartic potential is steeper than a quadratic one, more energy is needed to excite vibrations at higher temperature, where the atomic vibrations have larger amplitudes. This extra energy requirement explains the stiffening seen in the neutron scattering data.
Until now, the quartic potential had mainly been a theoretical curiosity, but it could be common in many oxide materials, says Caltech team member Chen Li. He and his colleagues expect quartic oscillations travelling through a crystal to interact with each other—unlike normal quadratic oscillations. Such interactions would disrupt heat flow, so the team speculates that a future material that maximizes this quartic behavior could make a good thermal insulator.
The team found that a quartic potential dominates the rigid unit mode that geometric models of NTE typically focus on. This does not imply that quartic oscillation causes NTE, but these potentials may affect the properties of scandium trifluoride and other similar materials. More theoretical work is necessary, the team says, especially since their computer simulations showed that the “rigid units” in scandium trifluoride are not as rigid as previously assumed.
Angus Wilkinson, who co-discovered NTE in scandium trifluoride, believes the team makes a good case for the importance of quartic potentials in this material. But he says they don’t explain why scandium trifluoride is “special.” Still, Wilkinson thinks the lack of rigidity the team found raises important questions about the theory of rigid unit modes in NTE.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- Benjamin K. Greve, Kenneth L. Martin, Peter L. Lee, Peter J. Chupas, Karena W. Chapman, and Angus P. Wilkinson, Pronounced Negative Thermal Expansion from a Simple Structure: Cubic ScF, J. Am. Chem. Soc. 132, 15496 (2010)
More Information
Bake, Shake, and Shrink (Focus story from 2004)