Plant Gas Valve under the Microscope
Plants suck water up from the ground through a system of narrow tubes that are periodically interrupted by thin, porous membranes. The membranes immobilize harmful gas bubbles, but they will open up like valves if the gas pressure becomes too high. To better understand this behavior, a team has used atomic force microscopy to measure the elastic properties of the porous membranes. Their results, reported in Physical Review E, support a model in which the operation of the membrane depends on how much it stretches under the pressure of a gas bubble. The insights might help in the development of microfluidic devices that mimic plants.
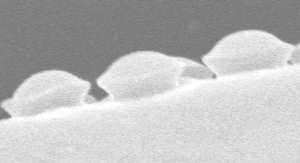
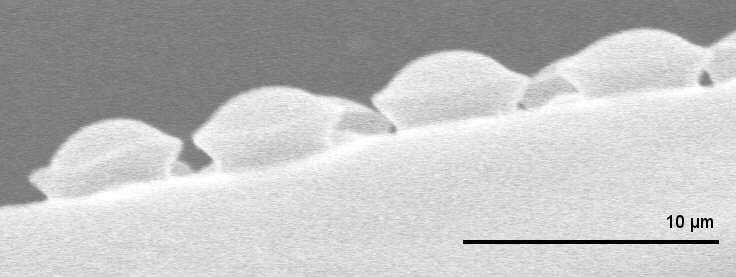
The vessels that carry water through a plant are called xylem. These vein-like tubes are made up of dead, hollowed-out cells that are typically a few centimeters long and to micrometers wide. Each xylem cell has a cap structure at each end, with an array of holes, or “pits,” for the water to flow through and into the next cell. Water flows upward thanks to a suction force produced through evaporation at the leaves. This pumping requires a negative pressure in the xylem that can sometimes cause dissolved gases in the water to form a bubble, which can fill most of a xylem cell and block the flow of water.
If a bubble fills up a xylem cell, there’s a danger that the gas will enter other cells and cause more bubbles to form. To trap the gas, each pit is covered with a thin, porous membrane whose pores are tens of nanometers across. Biologists assume that water on the opposite side of each pore forms a meniscus (gas-water interface) whose surface tension counters the gas pressure. However, the meniscus is predicted to break if the pressure reaches a certain threshold defined by the pore diameter. Previous measurements have confirmed that at higher pressures, gas can flow, so the membrane acts like a valve. But the observed pressures have not always been in agreement with the limit derived from the pore size [1].
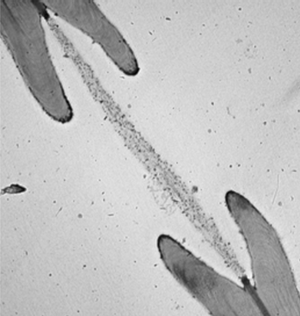
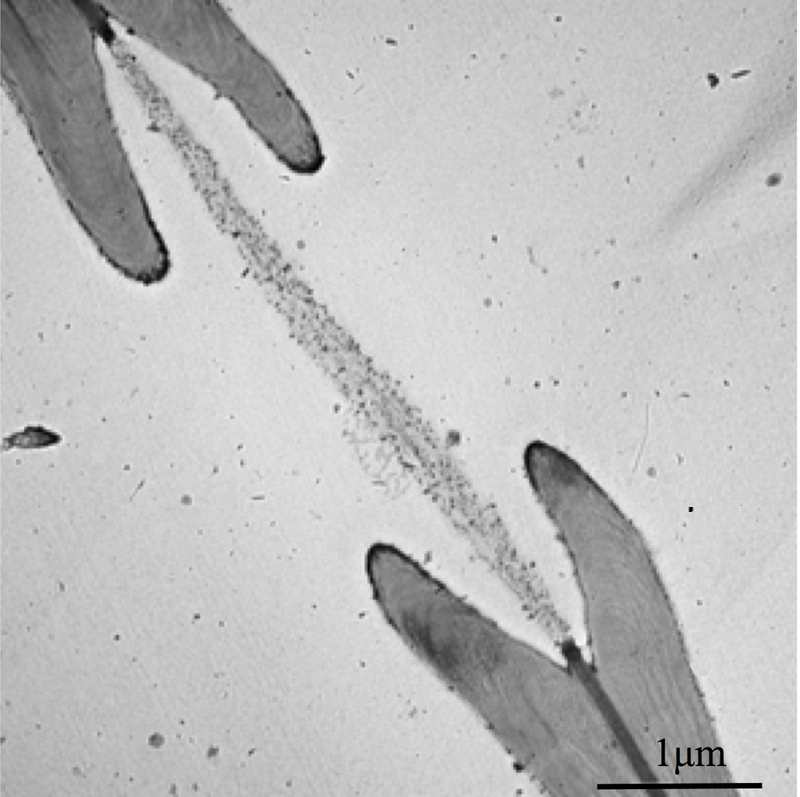
To better understand this gas trapping mechanism, Philippe Tordjeman of the University of Toulouse in France and his colleagues made a series of microscopic measurements of xylem and came up with a model that seems to explain the discrepancies from previous work. They began by imaging cross-sections of cottonwood branches with both transmission electronic microscopy and atomic force microscopy (AFM). Their data on the numbers of pits and the dimensions of pit membranes agreed with previous experiments.
The team then used the AFM probe to indent a pit membrane and measure the resistive force, or tension. From these data, they derived a stiffness for the membrane of about gigapascals and then predicted how much the membrane would bulge outward in response to gas pressure. To verify their predictions, they injected silicone into one end of several prepared branches, each time using a different injection pressure. When the silicone hardened, the researchers chemically dissolved the wood and were left with micromolds of the membranes, showing them stretched to different degrees, depending on the pressure, all in agreement with the predictions.
The team realized that the stretching of the membrane into a dome shape under pressure should expand the pores to a larger diameter, which they calculated to be around nanometers at the critical pressure, where gas begins flowing. Considering how the membrane area changes, they estimated the “relaxed” pore diameter to be just nanometers, showing that the discrepancies in previous measurements of pore sizes might be explained by membrane stretching.
This kind of valve could benefit efforts to mimic plants with microfluidic devices that, without moving parts, draw small quantities of fluid through channels, for medical [2] or chemical [3] analysis. Tordjeman says that porous membranes in the channels could control the flow rate automatically.
“The authors bring together a useful array of techniques to provide an unusually complete picture of the structure and mechanics of the border pit membranes,” says Abraham Stroock of Cornell University. However, he says there’s still no direct evidence that the pores are changing size through deformation of the membrane. Plants may have other tools, such as gel sealants, for regulating gas flow, so Stroock says this field needs “new experimental techniques with which to fully resolve the pore scale.”
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- B. Choat, “Pit Membrane Porosity and Water Stress-Induced Cavitation in Four Co-Existing Dry Rainforest Tree Species,” Plant Physiol. 131, 41 (2003)
- C. S. Effenhauser, H. Harttig, and P. Krämer, “An Evaporation-Based Disposable Micropump Concept for Continuous Monitoring Applications,” Biomed. Microdevices 4, 27 (2002)
- T. D. Wheeler and A. D. Stroock, “The Transpiration of Water at Negative Pressures in a Synthetic Tree,” Nature 455, 208 (2008)
More Information
An animation describing water transport in plants from the University of Nebraska