Water Wire
It flows in rivulets, puddles in depressions, falls from the sky; you can even buy it at Costco–three-dimensional, “bulk” water is everywhere. Now, in the 28 October PRL, researchers report a new configuration of a nearly one-dimensional column of water. Although similar forms of water are common in biology, they are rarely seen in the lab, so this liquid “nanowire” may soon reveal important properties of water at the molecular scale.
When using an atomic force microscope (AFM), water is usually the enemy. A typical AFM runs a pointy metal tip over a surface to map the lumps and ruts. As the tip hovers above each point, there’s an attraction to the surface; when it skims a bump, the tip is pulled towards it. The AFM is so sensitive that even a tiny drop of water from damp air gums up the tip, making it stick to the surface unnecessarily.
Wonho Jhe and his colleagues at Seoul National University in Korea were developing a more sensitive AFM that could detect atomic-scale bumps by scanning the surface more closely than a typical instrument does. But then something strange happened.
“We saw a very small kind of step-wise variation” in the pull, says Jhe. “It could be considered noise,” he says, but the group was curious. Could water still be a problem for their improved tip? In constant humidity, they systematically raised the AFM tip off the surface. The force gradient–a force related measurement, something like the stretchiness that distinguishes a weak rubber band from a strong one–didn’t go up continuously. Instead, it stayed constant for a bit, then jumped by 0.45 Newtons per meter, stayed steady again, then jumped twice more before falling to zero.
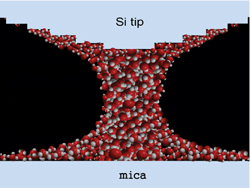
The effect disappeared when the humidity dropped below 2%, so the culprit was definitely water–behaving very oddly. The drop of water pulling on the AFM tip was miniscule, about liters, or ten yoctoliters. The tip stretched it into a one-dimensional thread, just a few molecules thick and tens of molecules tall. The group is not sure why the water pulls on the AFM with discrete, stepped levels in the force gradient, but it might result from individual water molecules alternately sticking, then sliding, then sticking again to the surface as the water column is stretched.
Researchers have in the past created one-dimensional water inside carbon nanotubes and columns of water several times thicker than Jhe’s with other techniques. But the new configuration is just 2.6 nanometers thick and appears in air at room temperature, much simpler conditions than in the past.
“It’s a good paper, it’s a dramatic paper, and probably extremely important,” says Eugene Stanley of Boston University. Biological cells use one-dimensional columns of water to transport ions through their membranes, he says, so the new technique may shed light on processes in cells. At the moment, Jhe’s group isn’t interested in the biological angle–his team is busy stretching, dragging, and playing with the one-dimensional water columns, trying to understand their structure.
–Kim Krieger
Kim Krieger is a freelance science writer in Norwalk, Connecticut.


