Sodium as a Green Substitute for Lithium in Batteries
This article is part of a series of pieces on advances in sustainable battery technologies that Physics Magazine is publishing to celebrate Earth Week 2024. See also: Q&A: Electrochemists Wanted for Vocational Degrees; Research News: Lithium-Ion “Traffic Jam” Behind Reduced Battery Performance; Q&A: The Path to Making Batteries Green; Research News: A New Cathode for Rechargeable Magnesium Batteries.
In the 1870 novel 20,000 Leagues Under the Sea, writer Jules Verne imagined a submarine powered by sodium batteries. That idea has resurfaced, as several battery companies have begun manufacturing sodium-ion batteries as greener alternatives to lithium-ion batteries. Sodium is just below lithium in the periodic table of the elements, meaning their chemical behaviors are very similar. That chemical kinship allows sodium-ion batteries to “ride the coattails” of lithium-ion batteries in terms of design and fabrication techniques. Recent demonstrations of sodium-ion batteries both for power tools and for automobiles have highlighted the rapid progress in the technology.
“Sodium-ion technology is really a clone of lithium-ion technology,” says Jean-Marie Tarascon from the College of France, who has worked for 35 years on battery technologies. Development of sodium-ion batteries has lagged behind that of lithium-ion batteries, but interest in sodium has grown in the past decade as a result of environmental concerns over the mining and shipping of lithium and its associated materials. Sodium is 1000 times more abundant than lithium, potentially reducing supply chains and lowering battery costs, Tarascon says. Other advantages of sodium-ion batteries include high power, fast charging, and low-temperature operation [1].
But there are also downsides to sodium-ion batteries, the top one being a lower energy density than their lithium-ion counterparts. Energy density has a direct bearing on the driving range of an electric vehicle, which means that sodium-powered cars may have trouble appealing to consumers who want a large vehicle that can go long distances. Lower energy density also affects the overall environmental impact of sodium-ion technology because more batteries are needed to supply the same amount of energy as the corresponding lithium-ion technology.
However, sodium-ion batteries are still improving, says Shirley Meng from the University of Chicago, who has been working on battery technology for 20 years. She says that the recent release of sodium-ion-powered products will accelerate development, as engineers will have data from real-world situations. “I have no doubt that the best sodium-ion batteries will work as well as lithium-ion ones in less than 10 years,” Meng says.
Batteries 101
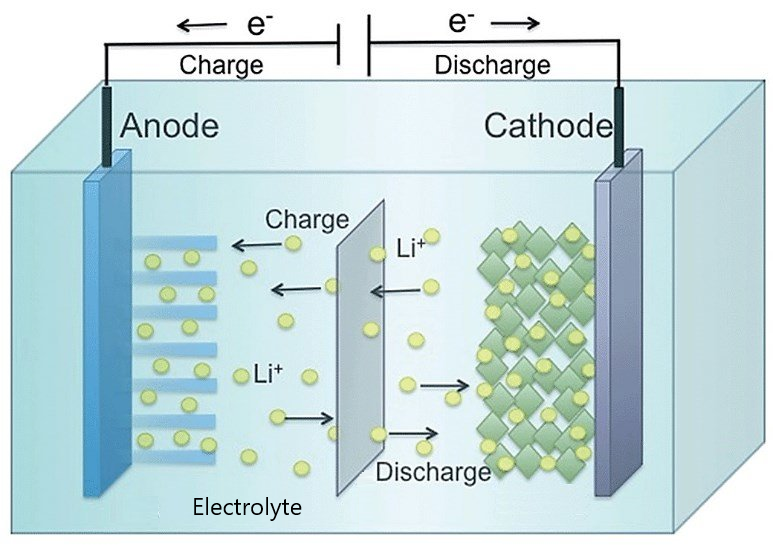
Developed in the 1980s and recognized by the 2019 Nobel Prize in Chemistry, the lithium-ion battery has become one of the most commonly used batteries in the world. It powers most phones and laptops, and it has driven the surge in electric vehicle production. Like most batteries, a lithium-ion battery consists of three main components: a positive electrode (cathode), a negative electrode (anode), and an ion-transporting medium (electrolyte) in between the two. There are various choices for the materials used for each component, but the most common design has an anode made of graphite (carbon); a cathode made of a lithium-containing metal oxide, such as lithium cobalt oxide or lithium manganese oxide; and an electrolyte that combines a lithium-based salt and an organic solvent.
When the battery is working (discharging), lithium ions come out of the anode and move through the electrolyte to the cathode where they are absorbed. When the lithium ions enter the cathode, a chemical reaction occurs that essentially “draws” electrons into the cathode from the connecting wire. During charging, electrons flow out of the cathode, freeing the lithium ions so that they flow back into the anode.
Lithium-ion batteries have a number of attractive attributes. First and foremost, they are rechargeable and have a high-energy density of 100–300 watt hours per kilogram (Wh/kg), compared to 30–40 Wh/kg for common lead-acid batteries. That high density means your laptop or cellphone can have a battery that lasts throughout the day without weighing you down. In the case of electric vehicles, a typical battery can weigh around 250 kg and supply around 50,000 Wh of energy, which is typically enough to drive 200 miles (320 km). Many environmentalists see this capability as our ticket for transitioning away from fossil fuels.
However, not everything about lithium-ion batteries is an environmentalist’s dream. The main issue involves the materials, since the extraction of lithium is resource intensive [3], and the mining of some of the metal ingredients is polluting [4]. There is also a lack of recycling infrastructure for today’s lithium-ion batteries, Meng says (see Q&A: The Path to Making Batteries Green). “The carbon footprint and the sustainability of the current way of making lithium-ion batteries is less than ideal.”
In addition to environmental concerns, the battery market is highly volatile, in part because the world has a limited number of lithium-rich regions. During the COVID pandemic, for example, the supply chain was cut off, and the price of lithium shot up. There are similar concerns over other lithium-ion-battery materials, such as nickel, copper, and graphite, which are also limited resources.
Lithium-ion alternatives include solid-state batteries (in which the liquid electrolyte is replaced by a solid one) and magnesium-ion batteries (in which magnesium ions replace lithium ions). Most of these options are still under development. And some of them also have issues concerning the availability of resources.
By contrast, sodium is abundant in seawater (although a more usable source is sodium ash deposits, which can be found in many regions of the world). And because sodium shares so much chemistry with lithium, sodium-ion batteries have been developing quickly and are already being commercialized. “Compared to other lithium-ion alternatives, I think sodium is at the forefront,” says Marcel Weil, who assesses the environmental impact of batteries at the Karlsruhe Institute of Technology and the Helmholtz Institute Ulm in Germany.
A Tale of Two Ions
Sodium-ion batteries are not new. Lithium and sodium systems were equally studied up until the 1980s. Interest in the two technologies diverged when researchers began to make breakthroughs in lithium-ion batteries. By the 1990s, research on sodium-ion batteries had largely halted. But some, including Tarascon, kept dabbling in the technology, even as they developed lithium-ion systems. In 2012, Tarascon helped relaunch sodium-ion research in France. His reasoning was that sodium appeared more sustainable. “It became obvious, to me at least, that the green technology would have a place in the future,” he says.
However, sodium and lithium atoms have differences, two of which are relevant for battery performance. The first difference is in the so-called redox potential, which characterizes the tendency for an atom or molecule to gain or lose electrons in a chemical reaction. The redox potential of sodium is 2.71 V, about 10% lower than that of lithium, which means sodium-ion batteries supply less energy—for each ion that arrives in the cathode—than lithium-ion batteries. The second difference is that the mass of sodium is 3 times that of lithium.
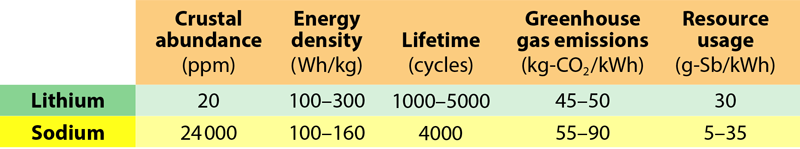
Together these differences result in an energy density for sodium-ion batteries that is at least 30% lower than that of lithium-ion batteries [1]. When considering electric vehicle applications, this lower energy density means that a person can’t drive as far with a sodium-ion battery as with a similarly sized lithium-ion battery. In terms of this driving range, “sodium can’t beat lithium,” Tarascon says.
The energy density is also a problem when considering the overall environmental impact of a battery. Weil and his colleagues performed a comparison of sodium-ion batteries to lithium-ion batteries, looking at a number of environmental factors such as greenhouse gas emissions and resource usage [5]. Although sodium-ion batteries do not require as many of our planet’s limited resources, they currently release more greenhouse gases during production than an equivalent energy’s worth of lithium-ion batteries. The reason is that larger quantities of materials need to be processed into batteries to produce the same amount of energy.
Weil says that this report provides a current snapshot, and in time, the environmental impact of sodium-ion batteries will likely improve. “We are convinced that they could have an even better overall performance than present lithium-based systems,” he says.
There are other differences between the two elements, some of which work in sodium’s favor. For example, sodium ions can travel faster through the battery materials than lithium ions, which might seem counterintuitive, given that sodium is heavier. Tarascon explains that a sodium ion has a diffuse electron cloud that allows it to slip between atoms more easily than a lithium ion, with its highly concentrated charge. The faster motion of a sodium ion can lead to higher power and faster charging in sodium-ion batteries.
Batteries Worth Their Salt
The current playbook for designing sodium-ion batteries resembles that of lithium-ion batteries. For the anode, most designs use “hard carbon,” which is like the graphite in lithium-ion batteries. The cathode options can be divided into three families of materials (metal oxide layers, polyanionic compounds, and Prussian blue analogs) that resemble those used for lithium. And the electrolyte is a similar cocktail of organic solvents.
Several research teams have tried to create sodium-based layered oxides for the cathode in an attempt to generate the high energy density that these compounds give lithium-ion batteries. Tarascon and his colleagues have taken a different strategy. They targeted a polyanionic compound—sodium vanadium fluorophosphate—as it seemed to be a promising material for making a high-power battery. And it appears that the bet paid off: last year Tiamat, a company for which Tarascon is a scientific advisor, produced a sodium-ion battery that is the first to be used in a commercial product—not a vehicle but a cordless power drill. The battery can charge in less than five minutes and can last a long time (over 5000 cycles), according to the company’s website.
Several large battery manufacturers have also announced sodium-ion projects that target the electric vehicle market. For example, CATL, a large Chinese battery company, announced last year that its first-generation sodium-ion battery—with an energy density of 160 Wh/kg—will be placed in an electric vehicle from the Chinese company Chery Automobile. Similar deals have recently been announced by the battery manufacturers HiNa and Farasis Energy, and several sodium-ion-powered vehicle prototypes have recently rolled off the assembly line. Meng calls these developments “very encouraging” as the companies will be collecting data under real-world driving conditions. “That information is vital for making the batteries better,” she says.
A Sociological Switch
But it may take some time before sodium-ion powered electric vehicles are widely available. One hurdle is economics. “The price of lithium has returned to a relatively low level, which makes sodium-ion batteries less competitive,” says a spokesperson from CATL. Moreover, they say, the lower energy density of sodium-ion batteries means the first target market will likely be smaller cars and two-wheeled vehicles.
In time, sodium-ion batteries will improve, but their driving range will never surpass the top-of-the-line lithium-ion batteries, Tarascon says. He imagines instead that sodium-ion technology will fill specific niches, such as batteries for smaller, single-person electric vehicles or for vehicles that have a range of only 30–50 miles (50–80 km). Weil agrees, but he says that society may have to change the way it views automobiles. “We cannot only point to the technology developers and say, ‘We need more efficiency.’ It’s even more important to stress that we need more ‘sufficiency,’ which is people being satisfied with a small car,” he says.
But whether sodium-ion or lithium-ion batteries come out on top, the world needs more battery-technology options if it is to reduce fossil-fuel consumption and combat climate change, Meng says. “If we always dream that one day a magic molecule is going to enable us to store solar and wind and use electricity when we need it, then I’m afraid that we will miss the golden opportunity to actually make some positive change.”
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- J.-M. Tarascon, “Na-ion versus Li-ion batteries: Complementarity rather than competitiveness,” Joule 4, 1616 (2020).
- P. Roy and S. K. Srivastava, “Nanostructured anode materials for lithium ion batteries,” J. Mater. Chem. A 3, 2454 (2015).
- W. Liu and D. B. Agusdinata, “Interdependencies of lithium mining and communities sustainability in Salar de Atacama, Chile,” J. Cleaner Prod. 260, 120838 (2020).
- S. H. Farjana et al., “Life cycle assessment of cobalt extraction process,” J. Sust. Mining 18, 150 (2019).
- J. F. Peters et al., “On the environmental competitiveness of sodium-ion batteries under a full life cycle perspective—a cell-chemistry specific modelling approach,” Sustainable Energy Fuels 5, 6414 (2021).
- J. Lindsay et al., “Sodium-ion batteries: A sustainable alternative to lithium-ion?” white paper, Minviro.