Towards Better Carbon Capture
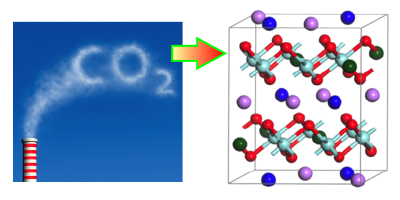
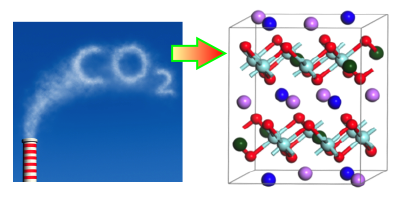
Concerns over climate change have stimulated research into ways of capturing carbon dioxide, so it can be sequestered underground. One technique involves using metal oxides that absorb over a specific range of temperatures and pressures. New computational work characterizes the behavior of a family of oxides, called alkali zirconates, and shows the effects that doping has on their absorption properties. The results offer a recipe for optimizing a zirconate absorber for carbon-capture applications.
Carbon capture technology is already used in some chemical processing industries, but it is not yet considered efficient enough to trap the emitted from power plants and other large emitters. One key hurdle is the cost of recycling the sorbent material after it has absorbed This typically requires heating it above a “turnover temperature,” at which point the material releases its into a storage tank. Researchers are searching for sorbent materials with low turnover temperatures that will reduce the amount of heating needed.
Recent work has shown that doping alkali zirconates can improve their carbon capture properties. To study this in detail, Yuhua Duan of the National Energy Technology Laboratory, Pennsylvania, and his colleagues performed first-principles calculations on sodium zirconate ( ), doped with either lithium or potassium. The computations described how the doping affects crystal structure, as well as electronic and phonon properties, which in turn influence the binding sites and capture reactions for The team showed that doping lowers the turnover temperature by an amount that depends on the type and concentration of dopant. The implication is that engineers could choose a dopant, or even a mix of different dopants, to obtain the optimum turnover temperature for a particular application.
This research is published in Physical Review Applied.
–Michael Schirber