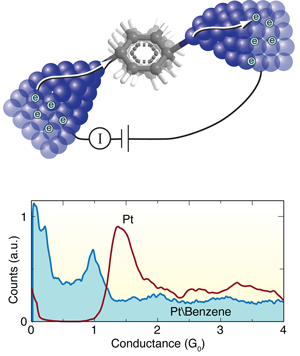
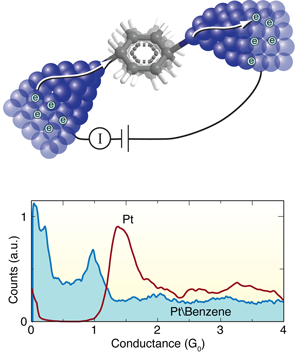
Benzene provides the missing link in molecular junctions
The search for ever smaller microelectronic circuitry demands that chip designers push basic components such as transistors and logic gates to the limits of miniaturization. To this end, researchers are keen to harness devices fabricated from single molecules attached to metal electrodes (Fig. 1, top). In the last decade, significant experimental progress has been made in constructing such molecular junctions and probing their electronic properties [1], [2], [3]. For example, the electrical conductance of a molecule depends on its structure, as well as the chemical link groups used to bond the molecule to the metal electrodes [4], [5]. In principle, this means that one could design a range of molecular electronic components simply by “playing with chemistry.”
For most junctions, the bonds between the atoms in the metal electrode and the molecule are not metallic thus the metal–molecule link creates a sizeable potential barrier across the junction. As a result, electrons typically tunnel across the molecule when a voltage is applied between the electrodes. A much higher conductance would be expected if the metal–molecule bond allowed electrons to travel ballistically, that is, without scattering, across the junction. This is the case for many carbon nanotube devices or single-atom contacts, which achieve the quantum of conductance ( )—the maximum conductance possible for a single electron channel. However, it has been quite difficult to achieve this level of conductance in single-molecule junctions. In the July 21st issue of Physical Review Letters Manabu Kiguchi, Oren Tal, and coworkers in the group of Jan van Ruitenbeek at the Leiden Institute of Physics in The Netherlands have shown for the first time that single-molecule junctions with a conductance of order can be formed by binding benzene directly to platinum metal electrodes [6]. The potential to make direct carbon–metal links will overcome a major barrier towards creating functional molecular based devices [7].
The Leiden group has pioneered techniques for forming and measuring single-molecule junctions. In this work, they start by breaking a notched platinum wire coated with benzene molecules at liquid helium temperatures [8]. As the wire is pulled apart, the notch thins out ultimately forming an atomic point contact. With reasonable success, they can get a benzene molecule to bridge the junction as it breaks, forming a molecular circuit with atomically sharp Pt electrodes. During this breaking process, they measure the current across the wire at a fixed voltage to determine the conductance as a function of the distance between the platinum tips. They form and break junctions repeatedly, thousands of times, and measure the conductance in the process so as to build a histogram that determines statistically significant junction conductance values (Fig. 1, bottom).
When a Pt wire is broken in a clean environment without any molecules, they see a peak in the conductance histogram at around corresponding to the conductance of a single Pt atom contact (Fig. 1, bottom). When they repeat this measurement in the presence of benzene, a broad conductance peak appears at around , while the signature of a clean Pt contact disappears. In this way, Kiguchi et al. can attribute the feature to the formation of a single Pt-benzene-Pt junction.
However, just seeing a peak in the histogram is not sufficient proof that a single-molecule junction has formed. In particular, the Leiden group wanted to make sure that the electrons that contribute to the current actually go through the molecule, rather than through a still existing Pt metal junction that is just perturbed by the benzene. To do this, they determine if the electrons that “flow” through the junction can excite vibrations in the Pt-benzene-Pt junctions using a technique known as inelastic electron tunneling spectroscopy (IETS)[9]. They form a stable junction and, holding the electrodes at a fixed separation, measure the current as a function of applied voltage.
If the electrons that go through the junction excite a vibrational mode in benzene, they will lose energy as they go through, resulting in an inelastic transfer process. This will occur only if the electron comes into the junction with an energy above the vibrational energy threshold. When such an inelastic process occurs, an additional pathway opens up for electronic transfer, resulting in an increased junction conductance. Thus the derivative of the current with respect to the voltage—the spectrum—will have a sharp rise around the vibrational energy threshold. This is what they observe when the applied bias voltage goes beyond 42 meV for low conductance (much less than ) Pt-benzene-Pt junctions.
Moreover, to show conclusively that the 42 meV vibrational mode is indeed due to benzene, and not due to inelastic scattering in the metal, they repeat this experiment using benzene containing instead of regular benzene. Increasing the mass of benzene decreases the energy threshold for exciting the same molecular vibrational mode, without changing any other transport characteristics significantly. They find that the vibrational mode shifts by about 2 meV in the benzene isotope, verifying that the excited vibration involves the entire benzene molecule and providing conclusive evidence that the electrons go through Pt-benzene-Pt junctions.
The next question they address, both experimentally and theoretically, involves the electronic nature of the Pt-benzene-Pt junctions. A quantum of conductance in a junction could signify that the system has a single channel (one “mode” of conduction) through which electrons propagate ballistically, with a unit transmission probability. This gives the system its maximal conductance, similar to what is observed in a single gold atom contact. However, it is also possible that the Pt-benzene-Pt junction has multiple conduction channels, each of which is only partly open, yet summed together having a transmission probability .
Kiguchi and coworkers probe the channel composition of their junctions by looking at the shot noise in their current measurements [10]. Due to the discrete nature of electron charge, current through any device fluctuates with the actual number of electrons traversing the device, which varies according to Poisson statistics. Furthermore, the noise depends on the number of channels available for conduction and their respective transmission probabilities. In a single channel device with unit transmission, shot noise is suppressed, however, if the transmission probability is not unity, then electrons can either be transmitted through or reflected from the channel. This leads to a fluctuation in current. Thus, by measuring the current noise through a device and fitting it to a theoretical expression, the number of channels and their transmission probabilities can be determined. From this analysis, the Leiden group determines that the Pt-benzene-Pt junctions can have up to three partly transmitting channels. The exact number varies from junction to junction and is probably controlled by the geometric details of the junction. This agrees with first principles calculations where they show that the benzene could be attached to Pt with either one, two or three carbon atoms bonded to one Pt on each side of the device.
Thus, although Pt-benzene-Pt junctions have a quantum of conductance, there are multiple channels contributing to the conductance and this depends on the exact details of the junction geometry. What makes this work stand out is that Kiguchi and coworkers have presented a new way to attach organic molecules to metal electrodes, by forming a direct metal–carbon bond, and have proven conclusively that their devices have a strong metal–molecule link. This enables them to overcome a major barrier in molecular based devices. The obvious next step would be to extend such measurements to bigger and longer molecules with terminal benzenes to show that benzene links can indeed be used as chemical “alligator clips.” However, the search for the best “link” cannot be deemed over; what they have shown with Pt-benzene links is that it is possible to fabricate a single-molecule device that is highly conductive. What still needs to be seen is if there is a link that would provide a unit transmission through an organic based device with a single conducting channel.
References
- M.A. Reed et al., Science 278, 5336 (1997)
- Wenjie Liang, Matthew P. Shores, Marc Bockrath, Jeffrey R. Long, and Hongkun Park, Nature 417, 725 (2002)
- Jiwoong Park, Abhay N. Pasupathy, Jonas I. Goldsmith, Connie Chang, Yuval Yaish, Jason R. Petta, Marie Rinkoski, James P. Sethna, Héctor D. Abruña, Paul L. McEuen, and Daniel C. Ralph, Nature 417, 722 (2002)
- Latha Venkataraman, Jennifer E. Klare, Colin Nuckolls, Mark S. Hybertsen, and Michael L. Steigerwald, Nature 442, 904 (2006)
- Y.S. Park, A.C. Whalley, M. Kamenetska, M.L. Steigerwald, M.S. Hybertsen, C. Nuckolls, and L. Venkataraman, J. Am. Chem. Soc. 129, 15768 (2007)
- M. Kiguchi, O. Tal, S. Wohlthat, F. Pauly, M. Krieger, D. Djukic, J. C. Cuevas and J. M. van Ruitenbeek, Phys. Rev. Lett. 101, 046801 (2008)
- G. S. Tulevski, Science 309, 591 (2005)
- R. H. M. Smit, Y. Noat, C. Untiedt, N. D. Lang, M. C. van Hemert, and J. M. van Ruitenbeek, Nature 419, 906 (2002)
- B. C. Stipe, M. A. Rezaei, and W. Ho, Science 280, 5370 (1998)
- H. E. van den Brom and J. M. van Ruitenbeek, Phys. Rev. Lett. 82, 1526 (1999)