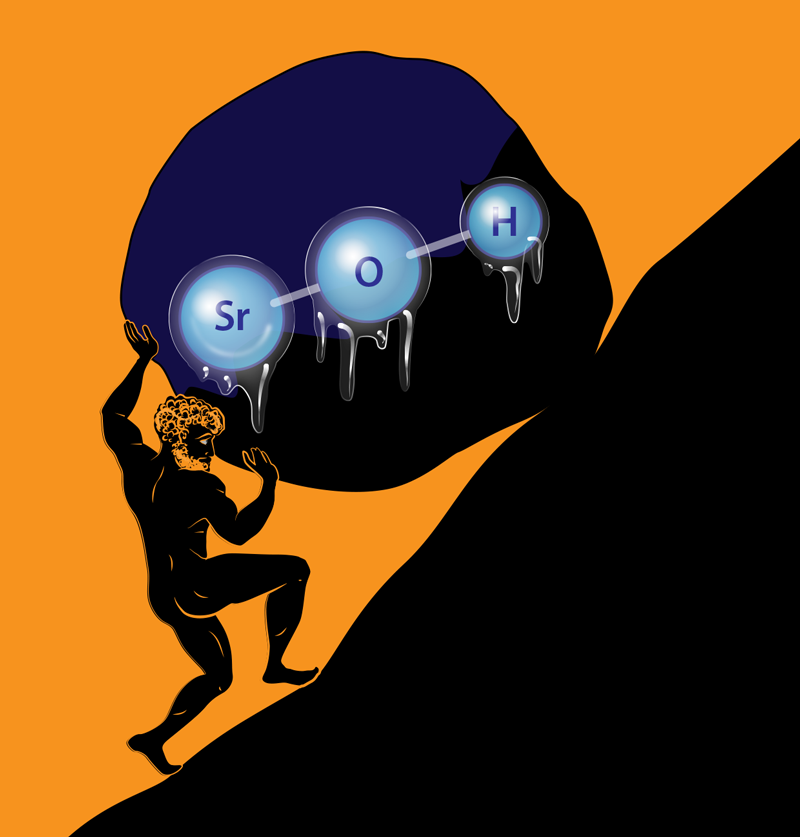
A Diatomic Molecule is One Atom too Few
Physicists considering a foray into the study of molecules are often warned that “a diatomic molecule is one atom too many!” [1]. Now John Doyle and colleagues [2] at Harvard University have thrown this caution to the wind and tackled laser cooling of a triatomic molecule with success, opening the door to the study of ultracold polyatomic molecules.
The technique of laser cooling [3], which uses the scattering of laser photons and the concomitant momentum transfer to bring atoms to a near halt, has revolutionized atomic, molecular, and optical (AMO) physics. Laser cooling and an important variant known as Sisyphus cooling [4] underpin three Nobel prizes in physics—for magneto-optical trapping (1997), Bose-Einstein condensation (2001), and the manipulation of individual quantum systems (2012)—and are crucial to a host of quantum-assisted technologies and fundamental physics measurements.
Since photons carry very little momentum and therefore reduce an atom’s velocity by just a small amount, a prerequisite for effective laser cooling is the ability to scatter thousands of photons. Thus laser cooling has predominantly been used only to cool simple atoms, whose electronic structure dictates that after a photon is absorbed, spontaneous emission places the atomic electron back into its original state, allowing the process to repeat nearly ad infinitum.
Spurred on by the possibility of another revolution in AMO physics when ultracold molecules become available [5], a brave group of researchers recently began work to achieve laser cooling of diatomic molecules, guided by a new proposal for how to deal with their complex structure [6]. Diatomic molecules, or “diatoms,” are challenging targets for laser cooling as their electronic structure is complicated by their rotational and vibrational degrees of freedom. When a diatom absorbs a photon from the laser, spontaneous emission can place it in any of a multitude of these rotational and vibrational states, whose transition frequencies no longer match that of the cooling laser. These so-called dark states are the bane of laser cooling, bringing the cooling process to a stop. Nonetheless, by carefully choosing molecules with unique properties—for example, those which contain an optically active electron that does not strongly participate in the molecular bonding—laser cooling of molecules has been successful, and it has culminated in the demonstration of magneto-optical trapping of SrF molecules [7].
Emboldened by these breakthroughs, Timur Isaev and Robert Berger [8] recently proposed a class of polyatomic molecules that appeared amenable to laser cooling. The crux of their proposal is that since SrF has many desirable properties for laser cooling, one might expect SrOH, where the OH acts as a pseudo-fluorine atom, to share in these properties—for instance, an optically active nonbonding electron. Building on this proposal and earlier work in their group, the Doyle team now reports the first observation of fast Sisyphus laser cooling of an untrapped polyatomic molecule [2]. Earlier work by Zeppenfeld and colleagues demonstrated slow Sisyphus cooling of a polyatomic molecule but required a trap to hold the molecules for many seconds [9].
Using a technique they pioneered, the authors produced SrOH molecules in a so-called cryogenic buffer-gas beam. They then selected those molecules with a transverse velocity slow enough for efficient laser cooling, corresponding to a transverse temperature of about 50 mK. Next they directed these molecules into a region where they interact with pairs of laser beams that are transverse to the molecular beam and are tuned near one of two optical resonances in the SrOH molecule. By measuring the transverse velocity distribution of the molecules after they have passed through the laser-cooling region, they inferred that not only did they cool the molecules, but they also observed Sisyphus cooling, which produced molecules with transverse temperatures of only 750 𝜇K.
In Sisyphus cooling, the molecules, like their doomed Greek eponym, are forced to climb a potential hill (Fig. 1) created by a standing wave of laser light, only to stumble when they spontaneously emit into a magnetic state that does not interact with the laser. A small magnetic field is used to bring the molecules back into the original state, so they can climb the potential hill again and continue to lose kinetic energy. In this manner, the molecules are quickly cooled; in fact, Doyle and colleagues observed that the molecules are cooled from 50 mK to 750 𝜇K by scattering only about 200 photons in 100 𝜇s—corresponding to an acceleration nearly 1000 times bigger than the acceleration due to gravity.
The fact that Sisyphus cooling can be effective with so few photons will likely be useful for the future of this field. Despite the ingenious ideas of Isaev and Berger [8], the extra complexity that comes with polyatomic molecules will almost certainly mean that the number of photons that can be scattered before the molecules spontaneously emit into a dark state will always be less than what is possible in diatoms and atoms. However, as demonstrated by the Doyle team, if Sisyphus cooling is used, a smaller number of scattered photons may be enough. Though, to realize the advantages of Sisyphus cooling, the molecules need to be precooled via another means, such as the buffer-gas-beam cooling employed by the Harvard team. This is more challenging for the forward direction of the beam, as opposed to the transverse cooling demonstrated by the Doyle team, since the molecules have a large forward velocity, but new techniques such as bichromatic force slowing [10] and optical deceleration are promising [11].
Despite this success, the authors are not resting on their laurels but are instead tooling up for their next experiments. Given that they measured that about 40% of the molecules were lost to dark states, they propose adding two additional lasers to recapture molecules from these states, which would allow them to scatter about 10,000 photons from SrOH. At this level, increasing the interaction time with the laser and cooling along the direction of the cryogenic molecular beam to slow it down may be enough to load the SrOH into a magneto-optical trap, where the collision properties of SrOH could be explored. The team has also proposed to continue their quest for larger molecules, by replacing the H in SrOH with more complex groups ranging from CH3 to (CH2)3CH3, which, while adding new degrees of freedom, should retain the necessary properties for laser cooling.
While it is certainly early in the exploration of polyatomic laser cooling, the future of this work is extremely exciting. If polyatomic molecules can be brought under the same level of control as we currently have over atoms, there would be enormous technological and scientific benefits. The extra degrees of freedom of these species yield convenient spaces for exploring ideas ranging from precision tests of physics, such as searches for variations in the fundamental constants, to quantum computation and simulation. Further, with cold chiral molecules, such as SrOCHDT, it could be possible to directly measure the energy difference, due to the weak force, between molecules that differ only by their handedness. Such a measurement may give hints as to why life seems to prefer organic molecules of one handedness over another [12].
Ten years ago, the idea that diatomic molecules could be laser cooled was borderline science fiction. Yet in 2014, SrF was laser cooled and trapped in a magneto-optical trap [7], and the present work reports laser cooling of a triatomic molecule. In the next ten years, perhaps our old warning that “a diatomic molecule is one atom too many” will need to be reconsidered as a diatomic molecule becomes one atom too few.
This research is published in Physical Review Letters.
Correction (2 May 2017): An earlier version of the article described a paper reporting Sisyphus cooling of a polyatomic molecule, but it didn’t make clear that a similar paper had previously reported the same type of cooling, albeit slow, for electrically trapped polyatomic molecules [M. Zeppenfeld et al., Nature 491, 570 (2012)]. This has now been corrected.
References
- This now-famous quote can be traced back to Arthur Schawlow, co-inventor of the laser, as he bemoaned “half-seriously” the difficulties of molecular spectroscopy. (Appeared in New Scientist, 22 October, 1981, p. 225).
- I. Kozyryev, L. Baum, K. Matsuda, B. L. Augenbraun, L. Anderegg, A. P. Sedlack, and J. M. Doyle, “Sisyphus Laser Cooling of a Polyatomic Molecule,” Phys. Rev. Lett. 118, 173201 (2017).
- D. J. Wineland, R. E. Drullinger, and F. L. Walls, “Radiation-Pressure Cooling of Bound Resonant Absorbers,” Phys. Rev. Lett. 40, 1639 (1978).
- J. Dalibard and C. Cohen-Tannoudji, “Dressed-Atom Approach to Atomic Motion in Laser Light: The Dipole Force Revisited,” J. Opt. Soc. Am. B 2, 1707 (1985).
- L. D. Carr, D. DeMille, R. V. Krems, and J. Ye, “Cold and Ultracold Molecules: Science, Technology and Applications,” New J. Phys. 11, 055049 (2009).
- B. K. Stuhl, B. C. Sawyer, D. Wang, and J. Ye, “Magneto-Optical Trap for Polar Molecules,” Phys. Rev. Lett. 101, 243002 (2008).
- J. F. Barry, D. J. McCarron, E. B. Norrgard, M. H. Steinecker, and D. DeMille, “Magneto-Optical Trapping of a Diatomic Molecule,” Nature 512, 286 (2014).
- T. A. Isaev and R. Berger, “Polyatomic Candidates for Cooling of Molecules with Lasers from Simple Theoretical Concepts,” Phys. Rev. Lett. 116, 063006 (2016).
- M. Zeppenfeld, B. G. U. Englert, R. Glöckner, A. Prehn, M. Mielenz, C. Sommer, L. D. van Buuren, M. Motsch, and G. Rempe, “Sisyphus Cooling of Electrically Trapped Polyatomic Molecules,” Nature 491, 570 (2012).
- M. A. Chieda and E. E. Eyler, “Prospects for Rapid Deceleration of Small Molecules by Optical Bichromatic Forces,” Phys. Rev. A 84, 063401 (2011).
- A. M. Jayich, A. C. Vutha, M. T. Hummon, J. V. Porto, and W. C. Campbell, “Continuous All-Optical Deceleration and Single-Photon Cooling of Molecular Beams,” Phys. Rev. A 89, 023425 (2014).
- M. Quack, “How Important is Parity Violation for Molecular and Biomolecular Chirality?,” Angew. Chem. Int. Ed. 41, 4618 (2002).