A Light Squeeze
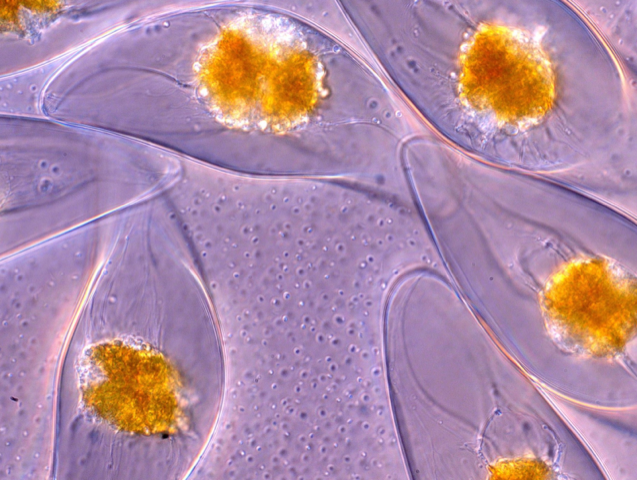
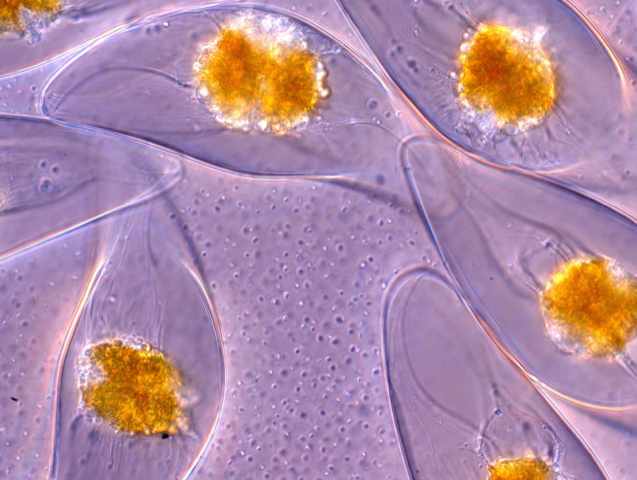
The glow or sparkle coming from bioluminescent marine organisms has fascinated observers on ships or the seashore since ancient times. A team of researchers has now elucidated the process by which mechanical stress triggers this light emission in single-celled organisms called dinoflagellates. By measuring the relationship between light intensity and the rate and amplitude of deformation for individual dinoflagellates, the team has developed a model that can pinpoint how different types of stress and deformation activate the glow.
Dinoflagellates are the most common source of bioluminescence in coastal waters. They are thought to use light emission as a defense strategy that alarms or dazzles predators [1], although it’s not clear if this is the sole role. As with other bioluminescent organisms, including fireflies, dinoflagellates produce light through a chemical reaction that ends with the molecule luciferin emitting a blue photon. The reaction is triggered by the electric potential created when pressure-sensitive calcium ion channels in the cell wall are stretched.
Previous deformation studies on single bioluminescent dinoflagellates used an atomic force microscope tip to indent the cell wall [2]. The results showed that the emission only switches on when the indenting speed exceeds a certain threshold, suggesting that the cell wall is a viscoelastic medium that can adjust to a slowly applied stress without triggering the pressure-sensitive channels. Raymond Goldstein of the University of Cambridge in the UK and his co-workers have now extended that study by exposing dinoflagellate specimens to two types of deformations that mimicked a breaking wave and the physical attack of a predator. Because this approach let them control the exact amount of deformation, and image it in detail, the researchers could connect their findings to a model of viscoelastic behavior in the cell walls.
Goldstein and colleagues studied the crescent-shaped dinoflagellate Pyrocystis lunula, which measures about 40 by 130 micrometers. Using suction, they picked up and held a single cell on a micropipette and subjected it—in the first set of experiments—to a jet of fluid from a second micropipette about 100 micrometers away. By tracking microparticles within the jet, the researchers could calculate the shear stresses exerted by the jet on the cell wall.
In a second set of experiments, the second pipette was pressed against the cell wall itself, deforming it mechanically. In both cases, the emission occurred as a flash that decayed within several tenths of a second, and the researchers could measure the amount and rate of deformation from microscope images. From the dependence of emission on deformation rate, the researchers fit the results to a simple model with three relevant timescales: the relaxation time of the viscoelastic cell wall, the decay time of the light-generating biochemical reaction, and the reset time for the cell to become ready for another flash.
Team member Maziyar Jalaal at Cambridge says that a mechanism like this, where the light production increases both for larger and faster deformations, makes adaptive sense, because the response then scales with the severity of the “threat.” He says the viscoelasticity of the cell wall acts as a “dynamical filter” that reduces the response when the deformation is more slowly applied, as, for example, in some shear flows.
Fluid dynamicist Howard Stone of Princeton University says that the work helps in bringing together previous research that focused on deformation either by macroscale fluid flow or by microscale direct pressure. Marine biologist Michael Latz of the Scripps Institution of Oceanography, California, hopes this new work will help explain variations in bioluminescent responses by clarifying “the as-yet unknown distribution of mechanosensors [ion channels] within the cell and how their activity is integrated into a whole-cell response.”
Latz also thinks this detailed study of bioluminescence could lead to a convenient method of flow monitoring. He imagines recording the light emission from dinoflagellates as a way to measure the shear stress, for example, in the flow around a moving dolphin, within a breaking wave, or inside a bioreactor.
Goldstein adds that “the very broad range of excitation stresses possible should have some bearing on the ecological significance” of bioluminescence, whose evolutionary origins and range of roles remain to be clarified. “This is just the beginning of what we hope will be an exciting line of investigation,” he says.
This research is published in Physical Review Letters.
(Correction 8 July 2020): The first caption has been corrected to properly describe the source of the bioluminescence within these cells.
–Philip Ball
Philip Ball is a freelance science writer in London. His latest book is How Life Works (Picador, 2024).
References
- S. H. D. Haddock et al., “Bioluminescence in the sea,” Annu. Rev. Mar. Sci. 2, 443 (2010).
- B. Tesson and M. I. Latz, “Mechanosensitivity of a rapid bioluminescence reporter system assessed by atomic force microscopy,” Biophys. J. 108, 1341 (2015).