Print Your Own Biological Implants
Ivan Minev dreams of a brain implant for treating epilepsy. Such a high-tech device would detect a seizure and immediately provide a drug dose, a pulse of electric current, or possibly even a bit of cooling to tamp down the chaotic brain signals. This technology sounds futuristic, but Minev, who works at the University of Sheffield, UK, and his colleagues are bringing it closer to reality. His team can now 3D print stretchy and flexible electrode arrays that can be implanted into rat brains and muscles for monitoring or influencing nerve signals. They have also built an easily adaptable device that can control heart cells in culture with both drugs and electrical signals. Minev discussed this progress at the online Materials Research Society conference last month.
Most biointerfaces, such as cochlear implants or prosthetic control circuits, rely exclusively on electrical signals, but biology has other ways to relay messages—through chemical signals and mechanical forces, for example. Minev wants to develop technology that “speaks more than just the electrical language,” he said. And it should be flexible in both the mechanical and design senses of the word—bendable like most biological tissues and also easy to adapt and prototype quickly, without the delays of traditional electronic fabrication techniques, such as lithography.
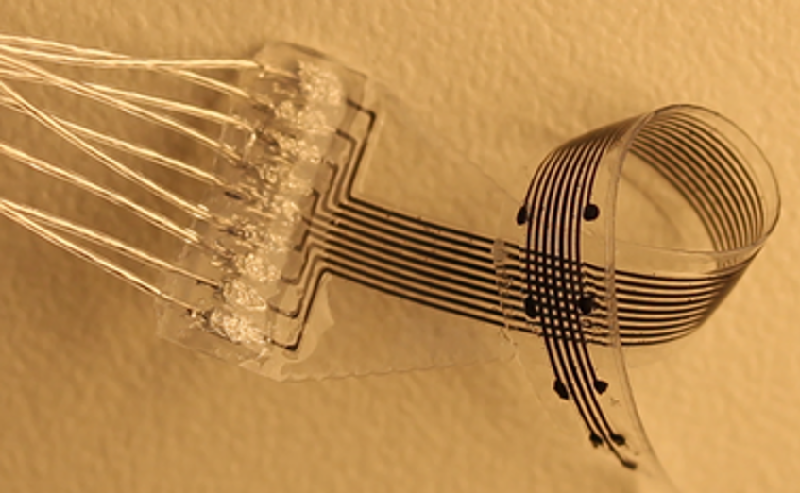
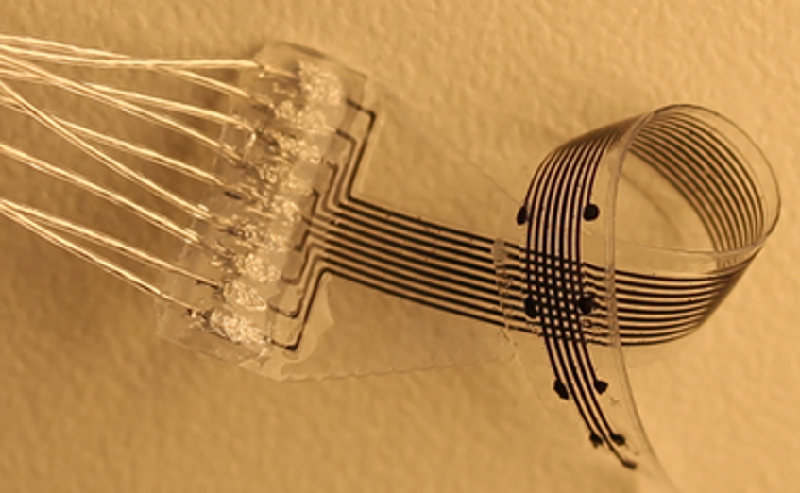
Minev and his colleagues have developed a 3D printing process and appropriate materials that allow them to produce small devices that are soft and flexible and that can include electrodes, optical fibers, and microfluidic channels. Although the features cannot be as small as those produced with lithography, the team is able to make some features as small as 120 m. The printing system also allows rapid redesign and prototyping.
As a demonstration, the team printed out a rubbery strip with an embedded optical fiber, microfluidic channel, and several electrodes. They then grew genetically modified heart cells on top of one end of the strip. These cells, which spontaneously emit periodic voltage pulses and automatically synchronize with their neighbors, had an “optogenetic” modification that allowed their electrical activity to be modified with light pulses. As the team recently reported, their device successfully integrated with the heart cells: the electrode monitored the voltage pulses, the fluid channel administered a pulse-frequency-increasing drug, and the optical fiber delivered light pulses that controlled the cells’ voltage pulse frequency.
Minev and his colleagues also recently demonstrated the first 3D-printed brain implant, which they placed into a rat brain and used to measure electrical signals connected with walking and standing. As part of this work, they were able to fabricate a variety of electrode array configurations for use in both stimulating and monitoring neurons in brains, spinal cords, and muscles of three different animals—rats, cats, and zebrafish. Minev said that the easy adaptability of the 3D-printed electrode structures may be important for neuroscience research. “It could make preclinical work more accessible for neuroscientists” who want to test new treatment concepts on animals. And the customizability could also be helpful for treating patients with personalized medicine, he said. “We hope to be able to print implants that are specific for a specific patient [and] their anatomy.”
–David Ehrenstein
David Ehrenstein is a Senior Editor for Physics Magazine.