Molecule’s Long-Lived Vibration in Superfluid Helium
The solvent in which a molecule is suspended can strongly influence the molecule’s motion. Now researchers have demonstrated that a molecule dissolved inside a superfluid helium nanodrop experiences very little effect from the solvent. The researchers measured, with femtosecond resolution, the intramolecular vibrations of an indium dimer (In2) in a helium nanodrop. They say that their method could be used to study molecules relevant for light-harvesting technologies, such as solar cells, that have been difficult to observe because of solvent effects.
A molecule’s intramolecular motions are known to affect how it interacts with light. Probing this motion is thus important for researchers designing solar cells, says Markus Koch of Graz University of Technology in Austria. To study a molecule’s vibrations, researchers typically take a molecule suspended in water, hexane, or methanol, and hit it with laser light. But there’s a problem with using these fluids: they can strongly interact with the molecule, changing how its atoms move. Koch and his team set out to see if swapping the traditional solvents for superfluid helium could alleviate this problem.
In earlier work, the team demonstrated that they could place a single indium atom inside a drop of superfluid helium [1]. They hit the atom with laser pulses to observe its motion, and they characterized the changes in the shape and stability of the void that formed around the atom as the atom was excited by the laser. Koch says that this characterization work was a key “prerequisite” for the new study, as it enabled the researchers to distinguish different motions of the system.
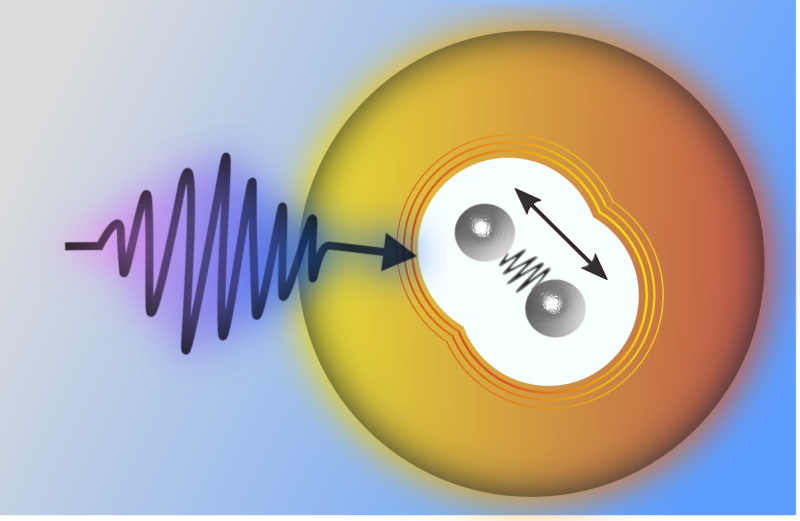
In their new experiments, the team used In2 instead of a single indium atom in the helium nanodrop. To package the molecules in the drops, they created an indium vapor inside a box and then moved a spray of superfluid helium drops through the box. An average drop passing through picked up two indium atoms, and the atoms then bonded to form In2.
To study the vibrations of this dimer, the researchers used femtosecond laser spectroscopy, a so-called pump-probe technique. First a laser pulse “pumped” the molecule, which excited it and set it vibrating. A few femtoseconds later, a pulse “probed” the molecule, causing it to emit an electron whose energy encoded information about the molecule’s vibrations. The team repeatedly bombarded a cloud of the drops with such pulses and measured the emitted electrons.
The electron energy oscillated in time, which provided a direct measure of the molecules’ vibrations. This oscillation faded away by 10 picoseconds (ps) but then reappeared—with the same frequency but one-fifth of the amplitude—after 145 ps. It then faded away and revived again with this same amplitude every 145 ps for the rest of the experiment.
Koch says that the initial decrease in the oscillation amplitude comes from a decrease in the coherence (synchronization) of the molecules’ vibrations caused by interactions with the superfluid helium. By the time the out-of-synch oscillations briefly re-synchronize at 145 ps, many of the drops have burst and have expelled their In2 molecules. So the oscillation amplitude—reduced by helium interactions—remains constant for the later revivals. The fact that the frequency is the same at late times as that observed in the first 10 ps indicates that the interaction with the helium is weak—if it were strong, the frequency would’ve changed. The 10-ps survival time of the initial oscillations also indicates a low interaction strength, as it is 10 to 100 times longer than that measured for other solvents.
Koch’s group is not alone in using superfluid helium drops as solvents for molecules, but they are the first to show that coherent vibrational spectroscopy is possible using this system, says Marcel Mudrich, an atomic physicist at Aarhus University in Denmark. He says that the long-lived vibrational motion that the team observes is likely a result of the void that forms around the molecule, which effectively decouples it from the solvent. Atomic physicist Frank Stienkemeier of the University of Freiburg in Germany agrees. He notes that this technique could allow for detailed studies of the electronic and vibrational properties of biological or light-harvesting molecules, which are “still far from being understood on a fundamental level.”
This research is published in Physical Review Letters.
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- B. Thaler et al., “Femtosecond photoexcitation dynamics inside a quantum solvent,” Nat. Commun. 9, 4006 (2018).