Complex Dance of Light-Seeking Algae in Light Gradients
Many photosynthetic microbes move in response to light. For example, the single-celled green alga Chlamydomonas reinhardtii swims toward moderate light to photosynthesize and away from intense light to avoid damage. Two longstanding questions about this light response regard how light-seeking cells move in a light-intensity gradient and whether this motion depends on cell concentration. Now, Aina Ramamonjy and colleagues at the French National Center for Scientific Research (CNRS) and the University of Paris have answered these questions [1]. The results could improve our understanding of how groups of photosynthetic organisms arrange themselves into dynamic patterns to control the amount of light that they receive.
In 1911, the botanist Harold Wager reported a seminal study [2] that launched the field of bioconvection, a collective phenomenon that results in self-organized structures and emergent flow patterns in suspensions of swimming microbes. The overall picture is that dense collections of microbes that are heavier than surrounding water but can swim against gravity self-organize into passively descending, cell-packed plumes flanked by actively ascending, cell-sparse populations.
This general picture has stood the test of time; many swimming photosynthetic microbes, including bacteria, algae, and protozoa, have been found to bioconvect at high cell concentrations. Bioconvection can emerge and persist for purely mechanical reasons. For instance, cells with a nonuniform mass distribution can have the mechanical stability needed to swim against gravity—a motion called gyrotaxis [3–5]. Most early studies investigated bioconvection associated with gyrotaxis. However, bioconvection can arise from a combination of other cues, which can compete with or reinforce each other. These cues (and their induced responses) include light-intensity gradients (phototaxis), gravitational forces (gravitaxis), chemical-concentration gradients (chemotaxis), and gas-concentration gradients (aerotaxis).
Photosynthetic algae inhabit dynamic environments where cellular physiology and fitness are governed by light-intensity gradients, in addition to fluid flow and nutrient availability. Given that the direction of increasing light intensity is inherently opposite to that of gravity, phototaxis and gravitaxis can be competing cues for algae depending on their gyrotactic stability. Algae can exploit this fact, swimming against gravity during the daytime to photosynthesize in well-lit surface waters and swimming with gravity at night to access nutrient-rich deep waters. Following initial attempts to observe these competing phototactic and gravitactic effects [6], scientists showed how light intensity and cell concentration can influence the bioconvective patterns formed by algae under spatially uniform light [7]. However, the movement of algae in a light-intensity gradient and the dependence of this motion on cell concentration remained poorly understood.
Ramamonjy and colleagues performed experiments using the alga C. reinhardtii—a model species for studying light-induced bioconvection owing to its strong phototactic and gravitactic abilities [8]. The team directed a beam of monochromatic green light at the center of a petri dish containing a thin layer of a C. reinhardtii suspension, exposing the algae to a light-intensity gradient (Fig. 1). The algae concentration varied from dilute to semidilute, the beam width ranged from 2.7to20mm, and the peak beam intensity ranged from 1to500W/m2.
The team began by using dilute suspensions, in which bioconvection was negligible. They measured the phototactic susceptibility 𝜒—quantifying the tendency for algae to migrate in a light-intensity gradient. These measurements were taken when the spatiotemporal distribution of the algae had reached a stationary state. The team plotted 𝜒 versus the light intensity and identified three distinct scenarios: 𝜒 was approximately zero (there was no phototaxis) for low intensities; 𝜒 was positive (the algae moved toward increasing intensity) for moderate intensities; and 𝜒 was negative (the cells moved toward decreasing intensity) for high intensities. These results showed that 𝜒 has a highly nonlinear dependence on the light intensity.
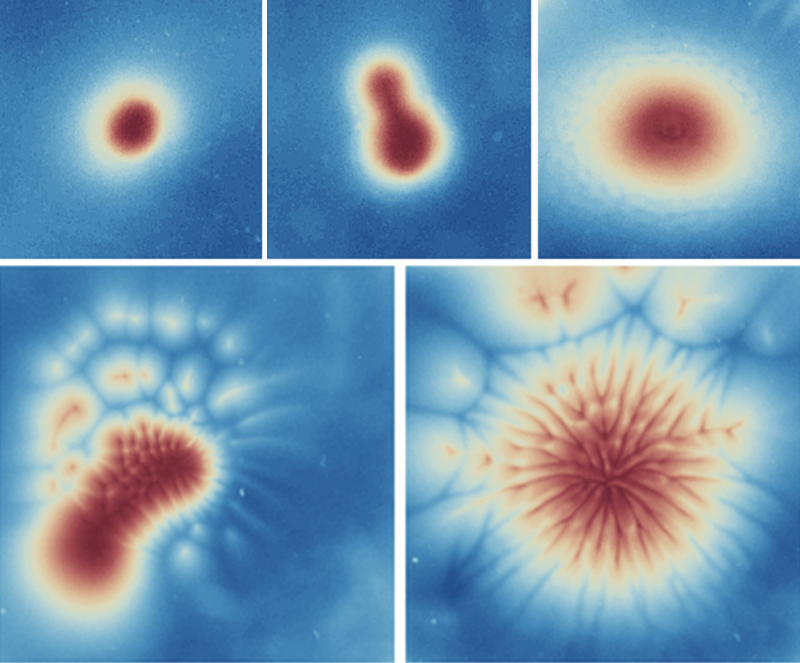
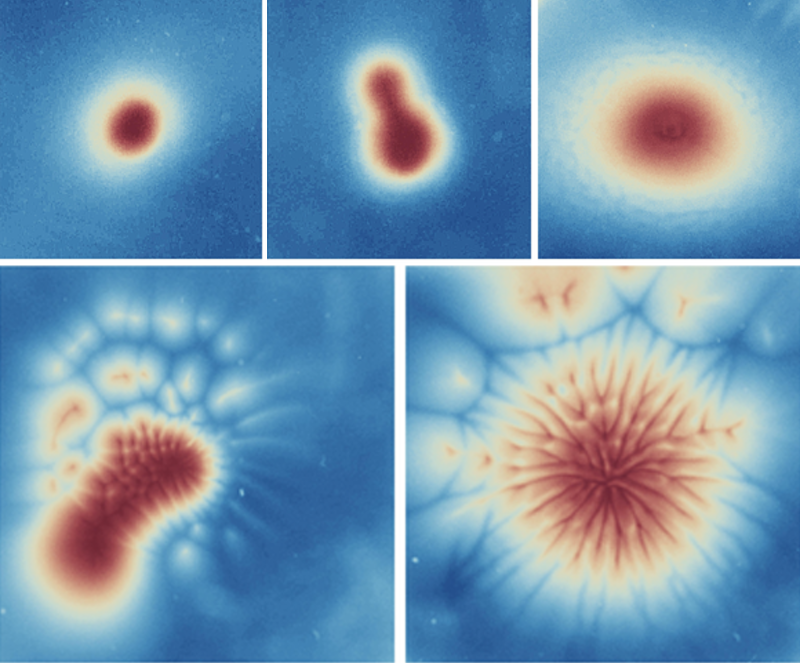
The team then used semidilute suspensions and observed that the algae formed five distinct spatiotemporal patterns, which they refer to as round, wave, dendrite, budding, and mixed (Fig. 2). The pattern that was produced depended on the light-beam width and on the system’s pseudo-Rayleigh number—a quantity that captures the relative importance of diffusive and convective transport processes. Looking at the bioconvective instabilities associated with these patterns, the team found that in the dendrite pattern, periodic thin extensions grew outward and then split in two; in the budding pattern, a bud-like structure grew from a central collection of algae; and in the mixed pattern, both these processes occurred simultaneously. The team explained the formation of these patterns using a semiquantitative gyrotactic model. They showed that this model can reproduce reasonably well the dependence of the pattern size on both the beam width and the pseudo-Rayleigh number.
Although the overall patterns Ramamonjy and colleagues found are qualitatively similar to those reported in Wager’s seminal work and elsewhere [2, 3, 8], they are the first to systematically study the bioconvective instabilities corresponding to those patterns. Given the ecological importance of microbial phototaxis (in tandem with gyrotaxis and gravitaxis), the team’s work is valuable in addressing a longstanding gap in our understanding of bioconvection in light-intensity gradients. From a biophysical perspective, the results pave the way for a holistic appreciation of the collective motion and emergent patterns associated with photosynthetic organisms in nature. These findings could be validated, even if only partially, through microbiologically motivated investigations that also account for chemotaxis or aerotaxis—two key determinants of microbial physiology and behavior in natural settings [9]. The team’s work might also help to classify collective patterns formed by a wide range of photosynthetic microbes across many ecosystems in response to huge variations in daily and seasonal light conditions.
These results bring us a step closer to tackling the crucial question: what is the role of light-induced bioconvection in microbial physiology? To quell lingering ambiguity about this role, future studies must account for ecologically relevant factors governing the dynamics of microbes in their natural habitats. For instance, changes to an alga’s cell shape and reorganizations of its subcellular structures can greatly alter gyrotactic stability [5, 10]. Accounting for such microbial processes will improve both macroscopic control and ecological understanding of bioconvection.
References
- A. Ramamonjy et al., “Nonlinear phototaxis and instabilities in suspensions of light-seeking algae,” Phys. Rev. Lett. 128, 258101 (2022).
- H. W. T. Wager, “On the effect of gravity upon the movements and aggregation of Euglena viridis, Ehrb., and other micro-organisms,” Phil. Trans. R. Soc. Lond. B 201, 333 (1911).
- M. A. Bees, “Advances in Bioconvection,” Ann. Rev. Fluid Mech. 52, 449 (2020).
- T. J. Pedley et al., “The growth of bioconvection patterns in a uniform suspension of gyrotactic micro-organisms,” J. Fluid Mech. 195, 223 (1988).
- A. Sengupta et al., “Phytoplankton can actively diversify their migration strategy in response to turbulent cues,” Nature 543, 555 (2017).
- J. O. Kessler, “Orientation of swimming flagellates by simultaneously acting external factors,” J. Phycology 28, 816 (1992).
- C. R. Williams and M. A. Bees, “A tale of three taxes: photo-gyro-gravitactic bioconvection,” J. Exp. Biol. 214, 2398 (2011).
- A. Javadi et al., “Photo-bioconvection: towards light control of flows in active suspensions,” Phil. Trans. R. Soc. A 378, 20190523 (2020).
- T. Sommer et al., “Bacteria-induced mixing in natural waters,” Geo. Phys. Res. Lett. 44, 9424 (2017).
- A. Sengupta et al., “Active reconfiguration of cytoplasmic lipid droplets governs migration of nutrient-limited phytoplankton,” bioRxiv (2021).