Droplets Come to Life
Droplets are an everyday phenomenon. We intuitively understand how they fall as rain and slither down a windowpane. Less familiar and intuitive is the behavior of so-called active droplets, which are fueled by chemical reactions. Nevertheless, active droplets are crucial in every cell of our bodies to organize myriads of biomolecules and their reactions. Leonardo Demarchi at Ludwig Maximilian University of Munich and his collaborators have now demonstrated that these active droplets can move autonomously or oscillate between confining walls [1]. Those behaviors could provide a clue about how life emerged from inanimate material.
Biological cells contain thousands of different types of biomolecules that jostle and react with each other. Despite that complexity, cells exhibit robust behavior and respond to countless external challenges. So far, we understand only minuscule parts of this intricate system and we know even less about how life-enabling complexity emerged spontaneously from inanimate material. However, in the past decades, it has become clear that weak physical interactions among biomolecules are a crucial part of the answer. Such interactions allow some molecules to stay together transiently while avoiding others, which can lead to the spontaneous formation of droplets whose composition differs from their surroundings. Although biochemist Alexander Oparin suggested such ideas a century ago [2], experimental corroboration arrived only recently [3].
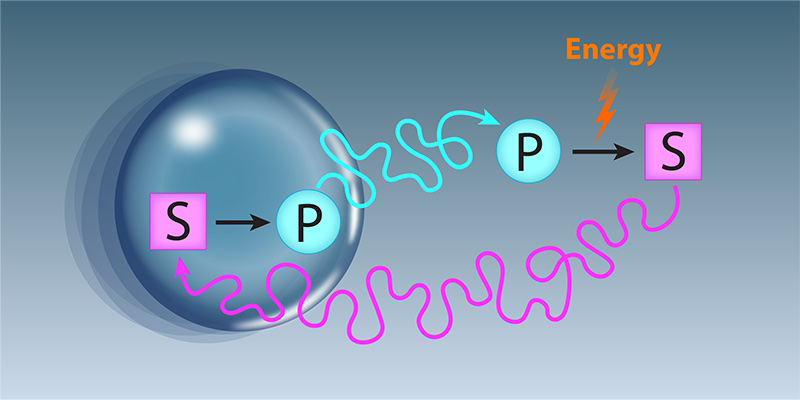
If droplets are so crucial, how can biological cells ensure that they form at the right time, at the right place, and with the right size? Chemical reactions in which enzymes control the conversion of “substrate” molecules into “product” molecules are a prime candidate for addressing that question because they control virtually all cellular processes (Fig. 1). Indeed, models suggest that these enzyme-controlled reactions can control droplet size [4] and even cause spontaneous division [5]. Building on this idea, Demarchi and his collaborators have demonstrated that catalytically active droplets can also move around. They investigate a minimal model combining phase separation with catalytically controlled chemical reactions, which they simulate numerically and treat analytically. Both approaches consistently demonstrate that the droplets are capable of self-propulsion.
Droplets form by phase separation, in which an initially homogeneous solution spontaneously forms two phases—a dense one and a dilute one. Phase separation originates entirely from physical interactions and is described by equilibrium thermodynamics. A droplet’s chemical contents are distinct from its surroundings and may concentrate enzymes so that a particular reaction takes place predominately inside the droplet. The droplet thus acts as a reaction crucible, which turns a substrate molecule present in the surroundings into a different product molecule. If the product molecule is converted back to the substrate molecule outside, a cyclic flux of material arises between the droplet and the surroundings, powered by external energy input coming, for example, from ATP in cells. Although such cyclic fluxes might seem like futile hamster wheels at first, they have been shown to affect droplets’ sizes and shapes. Essentially, that’s because large droplets are less efficient at converting substrate to product, whereas small droplets have prohibitively large surface energy for their volume. If substrate or product are heterogeneously distributed, droplets can also drift, akin to the way colloidal particles migrate through a viscous fluid in response to a gradient. The key contribution of Demarchi and his collaborators is to demonstrate that droplet drift can enhance the heterogeneity of substrate and product. The resulting positive feedback allows droplets to move continuously as if they were surfing on a self-generated wave.
The self-propulsion of active droplets has interesting consequences, particularly in confined geometries, such as inside a cell. When the droplets’ activity is weak, they are repelled from walls, so they seek a central position as far from the walls as possible. For stronger activity, droplets may overshoot the center and oscillate continuously between the walls, like a robot vacuum cleaner navigating a complex room. Sometimes the self-propulsion can be so strong that a droplet runs headfirst into the wall and gets stuck. All these behaviors result from the interplay between the droplet that consumes the substrate molecules in the surroundings and the replenishing mechanism that recycles the product molecules back into the substrate. The same interplay of chemistry and physics also mediates size control, which allows multiple droplets with the same chemical makeup to coexist and spontaneously divide to reach the optimal size.
In determining the behaviors of simple droplets so precisely, enzymatically controlled reactions could also be useful in technological processes. Chemists are beginning to tame such droplets in the lab to corral the immense potential for self-organization [6]. The systems’ built-in recycling is attractive because products and substrates are used continuously without generating undesirable waste.
Enzyme-enriched droplets possess many traits that we ordinarily associate with living creatures. First, they are well-defined entities separated from their surroundings. Second, they metabolize elements in their vicinity to control their composition and size, multiplying in times of abundance and dying in times of scarcity. Third, they move autonomously, reacting to obstacles in their environment to reach richer pastures. Despite these hallmarks of life, enzyme-enriched droplets lack both the internal complexity of structures that we ordinarily associate with life and the adaption needed for evolution. However, more complex droplets can exhibit internal structures [7], and it is likely that the imperfect copying of RNA and other large biopolymers could provide the malleability for evolutionary processes. Droplets could thus be a plausible step in the origin of life.
References
- L. Demarchi et al., “Enzyme-enriched condensates show self-propulsion, positioning, and coexistence,” Phys. Rev. Lett. 130, 128401 (2023).
- A. I. Oparin, The Origin of Life (Dover Publications, Mineola, New York, 1938)[Amazon][WorldCat].
- C. P. Brangwynne et al., “Germline P granules are liquid droplets that localize by controlled dissolution/condensation,” Science 324, 1729 (2009).
- C. A. Weber et al., “Physics of active emulsions,” Rep. Prog. Phys. 82, 064601 (2019).
- D. Zwicker et al., “Growth and division of active droplets provides a model for protocells,” Nat. Phys. 13, 408 (2016).
- C. Donau and J. Boekhoven, “The chemistry of chemically fueled droplets,” Trends Chem. 5, 45 (2023).
- S. Mao et al., “Phase behavior and morphology of multicomponent liquid mixtures,” Soft Matter 15, 1297 (2019).