Bringing Interferometric Imaging into the X-Ray Regime
Hanbury Brown and Twiss (HBT) interferometry [1] is a versatile technique widely used in various fields of physics, such as astronomy, quantum optics, and particle physics. By measuring the correlation of photon arrival times on two detectors as a function of the photons’ spatial separation, HBT interferometry enables the determination of the size and spatial distribution of a light source. Recently, a novel x-ray imaging technique based on the HBT method was proposed to image the spatial arrangement of heavy elements in a crystal or molecule by inducing those elements to fluoresce at x-ray wavelengths [2]. Now Fabian Trost of the German Electron Synchrotron (DESY) and colleagues—including some of those who first proposed the scheme—have implemented this technique, successfully demonstrating that the temporal correlation of fluorescence photons on a detector can be used to image the structure of emitters on a copper film [3]. This achievement marks a significant milestone toward extending HBT interferometry into high-resolution x-ray imaging, with the potential to image the structure and dynamics of isolated biomolecules without the need for crystallization [4].
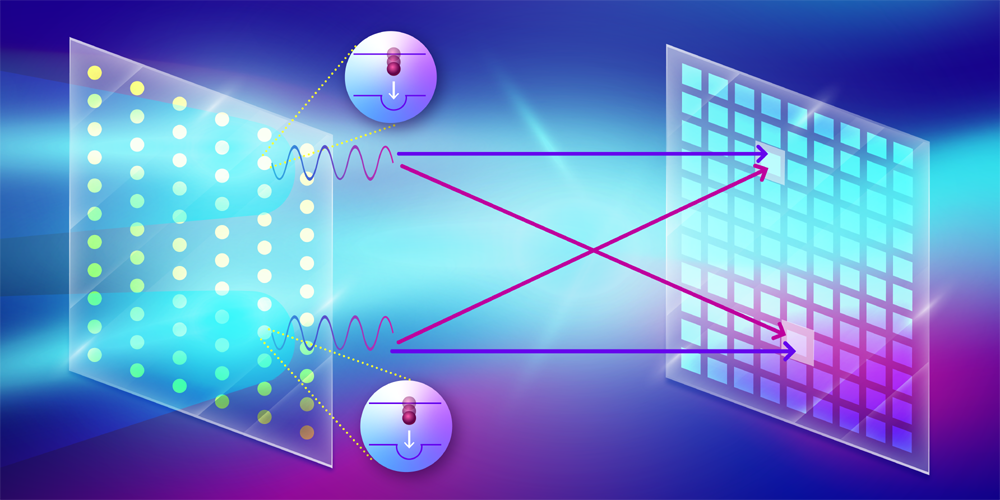
The HBT effect is a two-photon interference phenomenon that occurs when two indistinguishable photons emitted from different points within a source reach two different detectors. The indistinguishability condition is satisfied when the time interval between the two photons is within the coherence time of the light source. The interference effect, whether constructive or destructive, can be quantified using the second-order correlation function, or g(2), which describes the probability of detecting two photons simultaneously as a function of their spatial separation. If the arrival time is longer than the coherence time, this will lead to reduced contrast in the interference fringes in g(2).
Extending the HBT technique into the x-ray regime has been a challenge due to the low probability of x-ray excitation and the short coherence time of x-ray fluorescence, especially for heavy elements. Here the coherence time is given by the lifetime of the fluorescence states. For example, the lifetime of the electronic state of a copper atom with a vacancy in the K shell is less than 1 fs. The emission from two copper atoms will only be coherent or indistinguishable if the atoms are excited within that interval. The development of x-ray free-electron lasers (XFELs) has helped in this regard by making possible the generation of high-intensity x-ray pulses with femtosecond or even shorter durations [5]. These pulses can excite the K-shell electrons of heavy elements with a high probability and increase the chance of producing indistinguishable pairs of photons through x-ray fluorescence.
Trost and colleagues utilized such pulses to measure the intensity correlation of fluorescence photons emitted from a copper film. Conducting their experiment at the European XFEL facility in Germany, the researchers used a phase grating to diffract the incoming x-ray pulse and focus it onto two spots on a micron-sized copper foil. The incident x-ray photons had an energy of 9 keV, which was sufficient to ionize the K-shell electron of the illuminated copper atoms on the film. This ionization process created a short-lived excited state that primarily decayed via fluorescence emission, which the researchers measured using a detector specially developed at the XFEL facility. With one million pixels, each capable of single-photon detection, this detector can measure 1012 correlations between pixel pairs.
The team needed to address several challenges in order to implement x-ray imaging using this technique. One challenge was that the detector used in the experiment does not resolve the energy of the photons, meaning it cannot exclude contributions from other sources of radiation besides the desired K𝛼 emission. This nonselectivity would lead to a low signal-to-noise ratio. To address this issue, Trost and colleagues used a nickel filter to block elastically scattered radiation and copper K𝛽 radiation.
Another challenge was that the duration of the x-ray pulse was 10 times longer than the coherence lifetime of the K𝛼 emission, which reduces the contrast of the interference fringes. To improve the signal quality, the researchers recorded 58 million 2D fluorescence images in about 5 hours, enabled by the detector’s high readout rate and by the high repetition rate of the XFEL pulses. To ensure that the sample wasn’t damaged from the pulses, the copper foil was rotated such that each pulse illuminated a new area. Although the combined fluorescence image was isotropic and contained no structural information, the constructed g(2) revealed interference fringes reaching the third-order peaks, which is an improvement compared to previous studies that only measured the zero-order peak in g(2) [6, 7]. By using an iterative algorithm, the researchers successfully reconstructed the size (300 nm) and separation (860 nm) of the two excited spots on the copper film.
Given substantial improvement in spatial resolution, the technique could eventually enable single-particle imaging of biomolecules and catalysts at the atomic scale, and, with sufficient time resolution, characterization of their reaction dynamics. Continued advances in XFEL technology could also mean that the photon-hungry process of fluorescence excitation can be achieved with subfemtosecond pulses. For example, using intense, subfemtosecond x-ray pulses, it is possible to tailor the temporal fluorescence profile to be shorter than the lifetime of the fluorescing states [8]. To exploit the power of HBT interferometry for chemical imaging with elemental specificity, it is highly desirable to develop multicolor imaging using multiple detectors or detectors with energy-discrimination capabilities. Further development in this respect has the potential to revolutionize the characterization of important catalytic functions and of the associated structural changes in their native environments—such as metal-bearing clusters in metalloproteins [9]. Such characterization would provide unprecedented insight into these catalysts’ structure and activity, which is critical for the development of renewable energy sources.
References
- R. Hanbury Brown and R. Q. Twiss, “Correlation between photons in two coherent beams of light,” Nature 177, 27 (1956).
- A. Classen et al., “Incoherent diffractive imaging via intensity correlations of hard x rays,” Phys. Rev. Lett. 119, 053401 (2017).
- F. Trost et al., “Imaging via correlation of x-ray fluorescence photons,” Phys. Rev. Lett. 130, 173201 (2023).
- R. Neutze et al., “Potential for biomolecular imaging with femtosecond X-ray pulses,” Nature 406, 752 (2000).
- J. Duris et al., “Tunable isolated attosecond x-ray pulses with gigawatt peak power from a free-electron laser,” Nat. Photonics 14, 30 (2019).
- I. Inoue et al., “Determination of x-ray pulse duration via intensity correlation measurements of x-ray fluorescence,” J. Synchrotron Radiat. 26, 2050 (2019).
- N. Nakamura et al., “Focus characterization of an x-ray free-electron laser by intensity correlation measurement of x-ray fluorescence,” J. Synchrotron Radiat. 27, 1366 (2020).
- P. J. Ho et al., “Fluorescence intensity correlation imaging with high spatial resolution and elemental contrast using intense x-ray pulses,” Struct. Dyn. 8, 044101 (2021).
- J. Yano and V. Yachandra, “Mn4CA cluster in photosynthesis: Where and how water is oxidized to dioxygen,” Chem. Rev. 114, 4175 (2014).