How Proteins Control Embryonic Tissue Flow
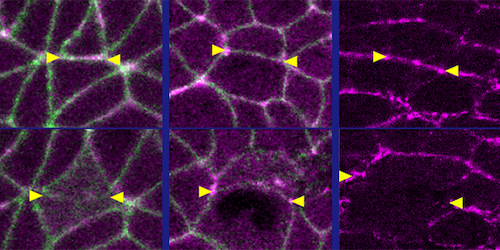
As an embryo develops, its epithelial tissues undergo dramatic deformations, transforming into intricate organism architectures. In vitro experiments suggested that a contractile protein complex known as actomyosin generates forces that can drive rapid tissue rearrangements. But researchers haven’t fully deciphered how actomyosin acts in living organisms. Now Marisol Herrera-Perez and colleagues at Columbia University have tackled the problem with in vivo “optogenetics” experiments, which use light to control the activity of actomyosin. The experiments show that actomysin behaves both as a force generator and as a mechanical-property regulator, toggling the tissue between solid- and fluid-like states [1]. The results show the potential of optogenetics for delivering a mechanistic understanding of tissue development, which could be a boon to tissue engineering, says Herrera-Perez.
The researchers investigated the Drosophila embryo, a model system for studies of embryonic tissue rearrangements. Specifically, they probed an early development stage when the embryo’s body-axis elongates. Using genetic techniques, they prepared embryos whose epithelia contained one of two types of light-sensitive proteins. When exposed to blue light, one protein type enhanced actomyosin’s contractility, while the other weakened it. The researchers then compared microscopic movies of the optogenetically modified embryos to those of wild-type ones at the same stage of development.
The results reveal that light activation and deactivation both reduced tissue flow—but through different mechanisms. Weakening of actomyosin’s contractility disabled flow by reducing the actomyosin-generated forces driving tissue rearrangements. Meanwhile, strengthening contractility increased those forces, but in an isotropic (nondirectional) way, which caused the tissue to get “stuck” in a solid-like state. These observations establish actomyosin’s dual role as force generator and gatekeeper of tissue fluidity, Herrera-Perez says. She adds that future research could apply similar approaches to more complex systems, such as mammalian embryos.
–Matteo Rini
Matteo Rini is the Editor of Physics Magazine.
References
- R. Marisol Herrera-Perez et al., “Tissue flows are tuned by actomyosin-dependent mechanics in developing embryos,” PRX Life 1, 013004 (2023).