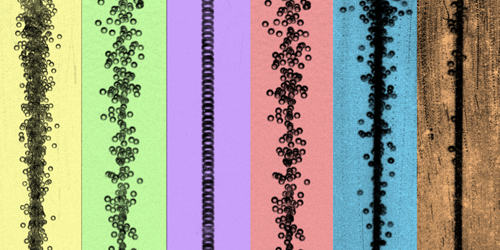
Straight Lines for Champagne; Wonky Ones for Cola
Roberto Zenit of Brown University, Rhode Island, is a bubble person, having spent his career studying various aspects of how these tiny spheres of gas behave. As a result, he says, he has “developed certain intuitions on how a bubble should behave under certain conditions.” One of those intuitions is that bubbles moving up through a carbonated liquid should follow a wonky path. That happens in a glass of soda but not in a flute of champagne. Zenit and his team now have an explanation for this bubbly difference [1]. The finding could help in understanding the behavior of bubbles in systems ranging from an ocean seep to a gin and tonic.
For their study, Zenit and colleagues performed experiments on carbonated water, beer, sparkling wine, champagne, and other fluids. They measured various physical and chemical properties of the liquids and their bubbles, including liquid density, bubble radii, bubble paths, and fatty-acid composition. They also performed numerical simulations of bubble chains moving through different fluids.
The experiments and simulations indicate that for small bubbles or for surfactant-free liquids, the wake structure of the rising bubbles is such that the vortices shed by the bubbles form a zigzag pattern, inducing lateral forces that destabilize the chain. For large bubbles or for surfactant-laden liquids, the shed vortices instead form a pattern that sucks the trailing bubble into the path of the first one, stabilizing the chain. Champagne has small bubbles but a high concentration of flavor-giving molecules (surfactants), so the chains are straight, Zenit says. For anyone interested in learning more, Zenit is happy to talk bubbles. Just make sure to bring along a glass of your favorite carbonated drink.
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- O. Atasi et al., “Presence of surfactants controls the stability of bubble chains in carbonated drinks,” Phys. Rev. Fluids 8, 053601 (2023).