Defects step aside to let battery current flow
Next-generation lithium ion batteries have turned out to perform better than expected. Naively, ion transport in these batteries should be blocked by crystal deformations in the electrode material, but these defects fortuitously clump together to leave open pathways. This defect sequestering is now explained for the first time in Physical Review Letters.
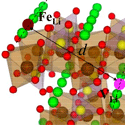
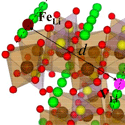
The lithium ion batteries found in many portable electronics are starting to be made with lithium iron phosphate ( , or LFP for short), which is safer and cheaper than alternative compounds. One of the unique properties of LFP is that lithium ions, which are released/absorbed by the material during charging/discharging, are forced to migrate along one-dimensional channels within the crystal. A misplaced iron atom can block the ion flow in a channel, but previous research has shown that, rather than appearing randomly inside the crystal, these iron-based defects congregate along just a few channels, so that ions can move freely along others.
To understand why this defect bundling occurs, Jaekwang Lee and his colleagues at Vanderbilt University and Oak Ridge National Laboratory in Tennessee focused on the movement of vacancies, or “holes” in the crystal structure, that facilitate ion migration. According to their calculations, the potential energy decreases whenever a vacancy comes into contact with an iron-based defect. The researchers detected this interaction with electron spectroscopy and then showed with statistical modeling that the frequency of these meetings increases—and thus the energy of the system decreases—when the defects align on a single channel. Their results imply LFP crystals should be synthesized slowly to allow defects time to congregate. – Michael Schirber