Deep Molecular Cooling
Laser-cooled atomic vapors have enabled research at the frontiers of physics for more than three decades, with a profound impact on precision spectroscopy, atomic clocks, and the study of Bose-Einstein condensates. The laser cooling process relies on a cycling transition in which millions of photons are absorbed or emitted between two energy levels of an atom, transferring momentum between the atom and the photons. By carefully tuning the laser close to an atomic resonance, the resulting momentum kick can be used to slow the atoms and take away their heat. It is natural to ask whether laser cooling can be generalized to molecules. The availability of ensembles of ultracold molecules (ultracold refers to an average kinetic energy corresponding to a temperature millikelvin) would enable new types of quantum gases, high-sensitivity measurements of fundamental constants, novel molecular clocks, and applications ranging from quantum computing to quantum chemistry.
Many of the characteristics that make cold molecules interesting derive from their complex internal structure, which can lead to a much richer phenomenology than for atoms. Unfortunately, this same structure may also be a liability, preventing the use of conventional laser cooling schemes. This occurs because in molecules it is difficult to single out two isolated levels. Once excited, molecules often decay to a vibrationally and rotationally excited state, from which they can no longer interact with the same laser field. Writing in Physical Review Letters, Isam Manai at the Laboratoire Aimé Cotton, France, and colleagues report on the culmination of their long-standing research efforts to cool ( microkelvin) molecules with lasers to the electronic, rotational, and vibrational ground state [1].
Through the mid-to-late 1990s, the belief that laser cooling of molecules would not be practical led most groups to focus on other approaches, e.g., using cryogenic techniques or slowing polar molecules in electric fields via the Stark effect. Though often effective, these methods could not access the ultracold regime of sub-millikelvin temperatures. But in 1997, laser-based cooling was back in the limelight when the group in Orsay presented a startling result: rather than directly cooling thermal molecules, ultracold molecules could be produced by photoassociation (i.e., laser-driven association) of previously cooled atoms. By this technique, the group demonstrated that molecular dimers of could be readily created at microkelvin in a magneto-optical trap [2]. Within months, ultracold and molecules were created using a similar approach [3]. While these were impressive results, such molecules, kinetically quite cold, were “internally” hot: they were not necessarily in the electronic ground state and, more importantly, they were mostly in excited vibrational (quantum number ) and rotational (quantum number ) states. Simply put, the challenge of creating molecules that were both kinetically and internally cold remained unanswered.
Several approaches were proposed to solve this problem. Researchers explored different photoassociation pathways and methods to transfer population of photoassociated molecules from highly excited rotational-vibrational states to the ground state ( , ). Sage et al. [4] formed RbCs molecules by photoassociation and then forced some of them into the vibrational ground state using stimulated emission [4]. In an elegant experiment at JILA in Boulder [5], ultracold atoms were magneto-associated into molecules, and then transferred into the ground state using a coherent Raman process involving a complex frequency-comb-based laser system. However, such methods are cumbersome and—perhaps with the exception of the experiment at JILA—could only create very diluted samples of cold molecules.
To develop simpler and more efficient schemes for rotational-vibrational cooling, researchers turned to the original ideas of Albert Kastler, who in 1950 was the first to propose cooling by optical pumping, an approach he named “luminorefrigeration.” Kastler suggested that, within the hyperfine manifold of an atomic system, optical pumping could be used to bring the atomic system into the lowest energy states [6]. His idea was that the population in higher-lying states could be excited and then made to decay preferentially into a ground state that is “dark,” i.e., not accessible by the optical excitation. The energy of atoms in excited states would thus be radiated away as light. Kastler’s idea is at the basis of deep cooling in optical molasses in which atoms are slowed at the intersection of six laser beams, one of the most widely used atom-cooling schemes. How can a similar principle based on optical pumping allow the cooling of the vibrational and rotational degree of freedom?
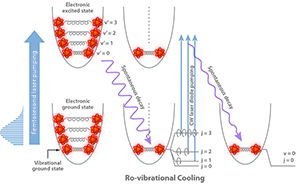
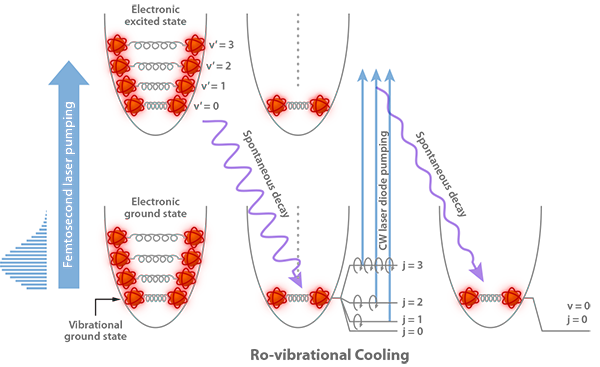
In 2008, the Orsay group took the first step by solving the vibrational part of the problem [7]. Their approach was to take advantage of the broad spectrum of short laser pulses. The authors carefully shaped the spectrum of a train of -femtosecond laser pulses, cutting away its high-energy side. This ensured that the pulses could not excite the vibrational level (it would be “dark”), while exciting all other ( ) vibrational levels from the electronic ground state into an electronic excited state. As the excited state spontaneously decayed, a fraction of the population would decay to and, eventually, through many pumping cycles, the population would accumulate there and the sample would cool vibrationally (see Fig. 1). In the laboratory, this worked wonderfully well. About of the population in the ten lowest vibrational states was cooled into the ground state in only a few tens of microseconds.
Addressing rotational cooling required solving another challenge. The vibrational cooling experiments of Wakim et al. [8], also based on optical pumping, showed that the rotational and vibrational degrees of freedom of the molecules could not be manipulated independently. As the molecules decayed from the excited electronic state, they did not necessarily return to the same rotational states from which they started. This resulted in the spreading of the rotational state population to higher rotational quantum numbers. In other words, while cooling vibrationally, some rotational heating was taking place. The realization of this important point was key to finding a solution for simultaneous rotational and vibrational cooling.
The Orsay group observed that rotational-vibrational cooling by impulsive laser schemes was not feasible. Vibrational cooling was enabled by the large vibrational energy level splittings, which allows tailoring a laser pulse capable of exciting a range of vibrational levels while creating a dark state for . In contrast, rotational levels are closely spaced, in particular for a heavy-atom diatomic molecule such as . Creating a spectral cutoff capable of making the rotational-vibrational ground state ( , ) a dark state for a short pump pulse would be impossible. The authors’ insight was to introduce instead a narrow-band, continuous-wave diode laser as an additional pumping laser. Since rotational levels are not equispaced they scale as , the laser frequency was swept to excite in succession the lowest ten excited rotational states ( to ) that were of importance in the initial, rotationally hot distribution. The combination of laser excitation and spontaneous decay drove the population from the high-lying rotational states down to , much in the way one sweeps dust on a staircase from the top step, step-by-step to the bottom (see Fig. 1). By combining vibrational pumping with rotational cooling, of the cold molecule population was transferred to the absolute ground state ( , ) within microseconds.
What is particularly elegant about the approach of Manai et al. is that it exploits exactly the process that was thought to preclude laser cooling of molecules—optical pumping. It remains to be seen how dense and large of an ensemble can be created using this scheme. Other competing approaches such as that pioneered at JILA will surely be explored, although their complexities are quite daunting when compared to the relatively simple scheme based on optical pumping. An important challenge will be to extend the Orsay approach to more complex molecular systems such as polar (heteronuclear) molecules. The availability of rotationally-vibrationally cold molecules may find intriguing applications in the test of one of the predictions of theories beyond the standard model of particle theory: a nonzero electron electric dipole moment (EDM). It has been speculated that the EDM could be detected from signatures in molecular spectra [9] if they could be measured with sufficient precision under conditions only achievable in ultracold molecules sitting in their rotational and vibrational ground state.
References
- I. Manai, R. Horchani, H. Lignier, P. Pillet, D. Comparat, A. Fioretti, and M. Allegrini, “Rovibrational Cooling of Molecules by Optical Pumping,” Phys. Rev. Lett. 109, 183001 (2012)
- A. Fioretti, D. Comparat, A. Crubellier, O. Dulieu, F. Masnou-Seeuws, and P. Pillet, “Formation of Cold Cs2 Molecules through Photoassociation,” Phys. Rev. Lett. 80, 4402 (1998)
- A. N. Nikolov, E. E. Eyler, X. T. Wang, J. Li, H. Wang, W. C. Stwalley, and P. L. Gould , “Observation of Ultracold Ground-State Potassium Molecules,” Phys. Rev. Lett. 82, 703 (1999); G. Gabbanini, A. Fioretti, A. Lucchesini, S. Gozzini, and M. Mazzoni, “Cold Rubidium Molecules Formed in a Magneto-Optical Trap,” 84, 2814 (2000)
- J. M. Sage, S. Sainis, T. Bergeman, and D. DeMille, “Optical Production of Ultracold Polar Molecules,” Phys. Rev. Lett. 94, 203001 (2005)
- K.-K. Ni, S. Ospelkaus, M. H. G. de Miranda, A. Pe’er, B. Neyenhuis, J. J. Zirbel, S. Kotochigova, P. S. Julienne, D. S. Jin, and J. Ye, “A High Phase-Space-Density Gas of Polar Molecules,” Science 322, 231 (2008)
- A. Kastler, “Quelques suggestions concernant la production optique et la détection optique d’une inégalité de population des niveaux de quantifigation spatiale des atomes. Application à l’expérience de Stern et Gerlach et à la résonance magnétique,” J. Phys. Radium 11, 255 (1950)
- M. Viteau, A. Chotia, M. Allegrini, N. Bouloufa, O. Dulieu, D. Comparat, and P. Pillet, “Optical Pumping and Vibrational Cooling of Molecules,” Science 321, 232 (2008)
- A. Wakim P. Zabawa, M. Haruza, and N. P. Bigelow, “Luminorefrigeration: Vibrational Cooling of NaCs,” Opt. Express 20, 16083 (2012)
- M. Kozlov and L. Labzowsky, “Parity Violation Effects in Diatomics,” J. Phys. B 28, 1933 (1995); J. J. Hudson, B. E. Sauer, M. R. Tarbutt, and E. A. Hinds, “Measurement of the Electron Electric Dipole Moment Using YbF Molecules,” Phys. Rev. Lett. 89, 023003 (2002); D. DeMille, “Sensitive Symmetry Tests with Diatomic Molecules,” Bull. Am. Phys. Soc. 49, 97 (2004)