Carbon Rings Allow Brief Electron Hook Ups
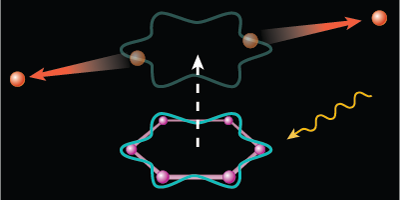
An electron pairing similar to that found in superconductors can arise from certain ring-shaped molecules, like benzene. When sufficiently excited, the organic molecules will eject two electrons that can briefly circle the molecular ring in unison before parting ways with each other. The authors, reporting in Physical Review Letters, dub this two-electron particle a Cooper pair, to draw connection to the so-named charge carriers in superconductors. Although the pairing here is too short-lived to provide any useful current, the discovery might help in the development of organic superconductors.
Molecules are ionized by light in the ultraviolet range. Usually only one electron is liberated in the process, but above a certain energy threshold, two electrons can be unchained from the molecule by a single photon. Previous investigations of double ionization have focused primarily on atoms and small molecules. But recently, Ralf Wehlitz of the University of Wisconsin–Madison and his colleagues found indications that double ionization may depend on the size or structure of the molecule.
In continuing this work, the group investigated several aromatic (ring-shaped) molecules, including four—benzene, naphthalene, anthracene, and coronene—that have one or more hexagonal carbon rings. When the team exposed their samples to extreme UV light, the hexagonally symmetric molecules exhibited an excess of double ionizations at photon energies around 60 eV. In these events, the two liberated electrons have just the right momentum to briefly hook together in a paired state that twirls around in a current loop defined by one of the carbon rings. The researchers found further evidence of the coupling in the energy spectra of the escaping electrons. – Michael Schirber