Remote Sensing of Surface Molecules
The invention of the scanning tunneling microscope (STM) [1] in the 1980s has allowed researchers an unprecedentedly close look at individual atoms and molecules on surfaces. In this technique, electrons quantum-mechanically tunneling from an atomically sharp metallic tip to a substrate allow researchers to obtain a highly resolved contour of the substrate: single adsorbed molecules appear as pronounced features—bumps—roughly resembling the outline of their frontier electronic states. This has provided physicists with direct insight into how solids reconstruct at surfaces where the material abruptly ends, which is important, for instance, in the fabrication of microelectronic devices. Surfaces are the interfaces by which materials can interact with their surroundings. Chemists use the STM to understand how surface structure gives rise to phenomena as diverse as catalysis and corrosion.
The STM is mind-boggling in its spatial resolution as fine as the size of atomic electron clouds, yet unfortunately it is chemically not very selective. That is, while each single molecule appears as a protrusion that may outline its overall shape, the STM does not directly indicate the number, identity, and arrangement of the atoms in it. From a chemical perspective, the latter would be crucial, as only precise stoichiometry and bond configuration determine a molecule’s identity and properties. To remedy this, many techniques (discussed below) have been developed so that, for instance, chemists can not only observe at which surface site molecules bind to undergo catalytic transformations, but they can also distinguish between similar-sized reactants and products.
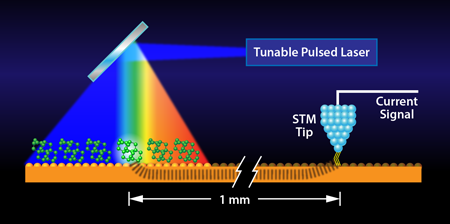
In a paper in Physical Review Letters [2], Ivan Pechenezhskiy and colleagues report an entirely new approach to chemical contrast in STM that relies on recording the light-induced vibrations of molecules with an STM tip that is millimeters away (see Fig. 1), much like a seismograph records the impact of earthquakes at a remote location, thereby allowing their characterization and classification.
Although progress on specific molecules has been made recently, STM cannot directly reveal the chemical bonding of molecules [3,4] (see also 15 August 2001 Viewpoint). This limitation arises because chemical bonds are just locations of charge density, which in their spatial extent do not differ very much for similar-sized atoms such as nitrogen and carbon (both second period elements), yet from a chemical perspective there is a huge difference [e.g., between hydrogen cyanide , which is highly toxic and almond-smelling, and acetylene , which is nontoxic and odorless]. This is also reflected by the electronic spectra of the species, but these generally cannot be measured by STM very precisely, because the underlying substrate also has electronic states that obscure them. Bond strength, the property that determines how stiff the “spring” is that connects adjacent atoms, however, is a direct indicator of which atoms are connected, and by extension, which molecule is present: the vibrational spectrum that arrives from the combinations of these springs can serve as a signature of a molecule, allowing its unambiguous identification. Being an all-electronic method, STM, however, provides no direct vibrational signature.
Ho’s group [5] found in 1998 that when an electron hops from the STM tip onto a molecule and then onto the substrate, it can impart a vibrational “kick” on the molecule in the process. This approach can reveal vibrational modes of the molecule, but it does not follow established selection rules, it is generally poorly resolved compared to infrared vibrational spectroscopy, and it is applicable only to particular adsorbate-substrate combinations. Alternatively, researchers including Ho [6], Morgenstern [7], myself [8], and others have attempted to combine optical excitation and STM in different ways. We all found that the presence of the tip right above the sample molecules reduces the options in such approaches. Namely, if a pulsed laser is used, the illumination will heat the tip and cause it to expand by many times the tunneling gap. This will smash the tip apex into both the molecule to be investigated and the substrate. Experimental schemes to avoid this either severely limit the utility of the method or allow one only to look for molecular hopping on the surface, which can also be caused by the electronic kick described above [9]. The latter can have some relation to vibrations excited in the molecule, but suffers from the same limitations as Ho’s original method.
Now, two University of California at Berkeley research groups, one with experience in STM, and the other, specialists in laser design and applications, have teamed up, conceived, and realized an alternative approach [2]. They set up a highly stable, pulsed laser source and pointed it on a spot of a gold surface covered by a particular molecule. Rather than placing their STM tip at the laser spot, they position it on the substrate approximately one millimeter away (i.e., some 1 million molecular diameters). Nevertheless, when tuning the frequency of the laser light to resonate with a molecular vibrational mode, they find a signal in the STM current. This they attribute to the molecule absorbing the light, becoming vibrationally excited, and then dissipating the vibrational energy to the substrate. Remarkably, on the particular molecules they chose—tetramantanes—the resonance turns out to be extremely sharp, allowing for single wave-number resolution in their vibrational spectroscopy, far better than in prior STM-based approaches.
The finding of Pechenezhskiy et al. [2] offers a fundamentally novel way of recording vibrational excitations at surfaces with unprecedented spectral resolution. However, it does not yet harness the particular strength of STM—the ability to resolve individual molecules separately. Because of the remote ( away) optical excitation, the STM cannot practically image the set of molecules that cause the substrate excitation in the first place. Moreover, the set of molecules excited is very large because it is determined by the submillimeter diameter of the laser spot and not the angstrom sharpness of the tip. It is expected that with better understanding of how the excitation travels through the substrate, e.g., in the form of local heating, vibrational excitation, plasmons, or through bound electronic states, researchers may be able to recover the exceptional spatial resolution of the STM. For now, the ground has been shaken, even if only at the nano level, and it has been recorded at high fidelity.
References
- Press Release: The 1986 Nobel Prize in Physics, http://nobelprize.org/nobel_prizes/physics/laureates/1986/press.html
- I. V. Pechenezhskiy, X. Hong, G. D. Nguyen, J. E. P. Dahl, R. M. K. Carlson, F. Wang, and M. F. Crommie, “Infrared Spectroscopy of Molecular Submonolayers on Surfaces by Infrared Scanning Tunneling Microscopy: Tetramantane on Au(111),” Phys. Rev. Lett. 111, 126101 (2013)
- L. Gross, F. Mohn, N. Moll, B. Schuler, A. Criado, E. Guitian, D. Pena, A. Gourdon, and G. Meyer, “Bond-Order Discrimination by Atomic Force Microscopy,” Science 337, 1326 (2012)
- L. Gross, N. Moll, F. Mohn, A. Curioni, G. Meyer, F. Hanke, and M. Persson, “High-Resolution Molecular Orbital Imaging Using a p-Wave STM Tip,” Phys. Rev. Lett. 107, 086101 (2011)
- B. Stipe, M. Rezaei, and W. Ho, “Single-Molecule Vibrational Spectroscopy and Microscopy,” Science 280, 1732 (1998)
- S. W. Wu and W. Ho, “Two-Photon-Induced Hot-Electron Transfer to a Single Molecule in a Scanning Tunneling Microscope,” Phys. Rev. B 82, 085444 (2010)
- M. Mehlhorn, H. Gawronski, L. Nedelmann, A. Grujic, and K. Morgenstern, “An Instrument to Investigate Femtochemistry on Metal Surfaces in Real Space,” Rev. Sci. Instrum. 78, 033905 (2007)
- L. Bartels, F. Wang, D. Moller, E. Knoesel, and T. Heinz, ”Real-Space Observation of Molecular Motion Induced by Femtosecond Laser Pulses,” Science 305, 648 (2004)
- K. Motobayashi, Y. Kim, H. Ueba, and M. Kawai, “Insight into Action Spectroscopy for Single Molecule Motion and Reactions through Inelastic Electron Tunneling,” Phys. Rev. Lett. 105, 076101 (2010)