The Calisthenics of Surface Femtochemistry
It has long been a chemist’s dream to catch a chemical reaction in the act. In a chemical reaction, reactant atoms or molecules pass over energy barriers, through transition states, and eventually convert into new chemical products. Chemists would like to observe and interfere with this process in mid-course and thereby control its outcome. The development of lasers that produce pulses of light just a few femtoseconds long has allowed researchers to initiate and track chemical reactions in the gas phase. Typically, one femtosecond pulse initiates the reaction through excitation of the reactant to a new electronic state, which drives the reactant’s nuclear motion. Another pulse, time-delayed from the first, then monitors the nuclear motion as the excited reactant decays or a new product forms. This approach gave rise to the field of femtochemistry and earned Ahmed Zewail (1946–2016) the 1999 Nobel Prize in Chemistry [1].
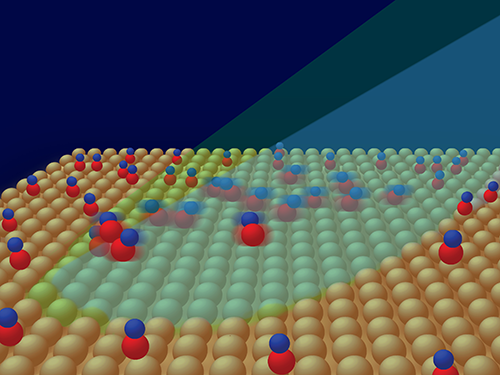
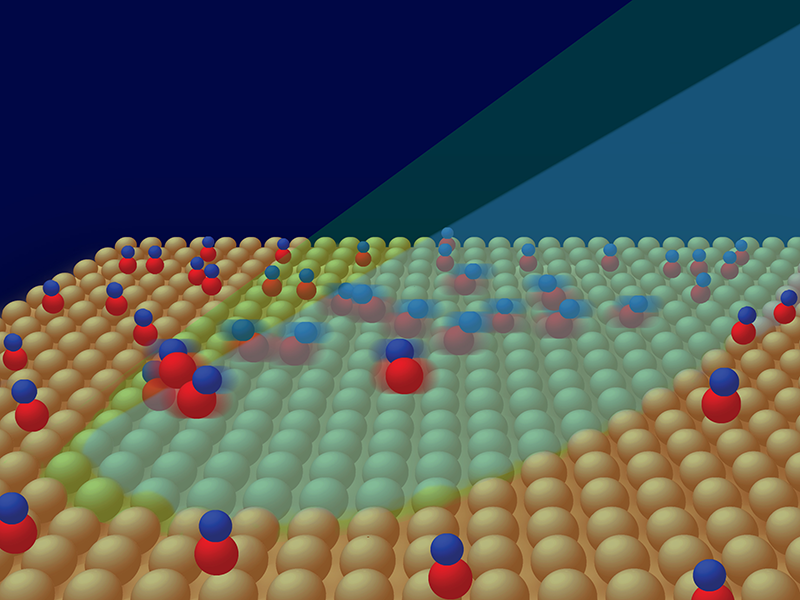
Many important chemical reactions, however, occur in more complex environments where molecules experience interactions, such as in condensed-matter systems. Moreover, there has been a strong impetus to extend methods of femtochemistry to surfaces because they can serve as models for catalysis. Catalytic substrates are used to lower energy barriers for chemical reactions of adsorbed molecules and thereby reduce the cost of producing economically valuable products, such as fertilizers, fuels, and pharmaceuticals. Yoshiyasu Matsumoto from Kyoto University, Japan, and co-workers now report [2] on one of the most basic chemical processes on a surface—the desorption of carbon monoxide (CO) molecules from a copper surface, driven by femtosecond-laser heating of its electrons (Fig. 1).
When a femtosecond “pump” laser pulse interacts with a surface, an adsorbed molecule can be energized in two ways: directly, by light-induced charge transfer from the surface to the molecule; or indirectly, through the heating of surface electrons [3]. The latter method, which Matsumoto and colleagues employ, is more general because hot electrons can transfer their energy to any adsorbed molecule, regardless of its electronic structure, through a process called electronic friction [4]. Electrons in a semiconductor or metal have a much smaller heat capacity than their host lattice. This means that, when a femtosecond laser pulse excites these materials, electrons can quickly reach temperatures exceeding 5000 K. These extremely hot electrons subsequently transfer their energy to the vibrational modes of the adsorbed molecule and the lattice, allowing chemical reactions between the two to be initiated [5].
To track the dynamics provoked by the transfer of energy from hot electrons, such as the breaking or rearrangement of the chemical bond between the molecule and the surface, one needs a “meter stick” to “report on” the process. This can be accomplished by probing the change in the molecule-surface electronic structure, using methods such as photoemission spectroscopy [6] and x-ray absorption spectroscopy [7]. But these methods are demanding: the first requires relatively long-lived (tens of femtoseconds) electronically excited states, which are rarely found, whereas the second entails using ultrafast x-ray laser sources that exist only in a few specialized user facilities. The approach taken by Matsumoto and co-workers has none of these drawbacks, relying on a broadly applicable and accessible time-dependent measurement of the vibrational spectrum of the excited complex.
The approach incorporates phase-sensitive detection—to precisely follow the minute frequency changes of a molecular vibration—into a time-resolved sum-frequency generation (SFG) spectroscopy experiment. In this type of experiment, two femtosecond pulses of photons with different frequency interact with the nonlinear electrical polarizability of the surface and combine to form a third pulse whose photon frequency is the sum of the first two. With one incident pulse tuned to the desired molecular vibration, which in Matsumoto and co-workers’ case was the internal stretching vibration of the adsorbed CO molecules, the SFG signal gains resonance enhancement and simultaneously probes the frequency of the vibration. Similar SFG experiments have been used previously to study the desorption of CO from a metal surface [8]. But the combination of phase-sensitive detection with SFG has allowed Matsumoto and colleagues to garner information on how hot electrons interact with molecular vibrations to affect the desorption process. In other words, the researchers used the phase of the CO stretching vibration as a “reporter” [9], with a period of about 13 fs, of the kicks and shoves delivered by hot electrons to the external motions of the CO molecule during desorption.
When a CO molecule is adsorbed on a copper surface it can no longer move as it does in the gas phase. The molecule forms a bond with the surface (Cu-C bond) that hinders its translation and rotation; that is, its translation and rotation modes become external vibrational modes. Exciting these modes causes translation, rotation, and eventually the breaking of the Cu-C bond and desorption of the molecule. Matsumoto and co-workers cool their sample to 100 K to suppress these motions and then use the femtosecond laser pump pulse to excite them through surface-electron heating. In response, the CO stretching vibrational frequency initially red shifts, and after several hundred femtoseconds it blue shifts. The researchers interpret these changes as a rapid energy transfer from hot electrons to the hindered rotation mode, followed by a slower excitation of the external Cu-C stretching mode. This interpretation is based on calculations of the potential energy surface for CO on copper and numerical simulations based on the Langevin equation, which describes the time evolution of a subset of degrees of freedom. Apparently, hot electrons pump energy into the rotation and stretching degrees of freedom with different efficiency. This occurs in such a way that when the molecule reaches the transition state, these degrees of freedom evolve into free-molecule rotation and translation without achieving mutual equilibrium as commonly assumed in transition-state theories of chemical reactions.
Matsumoto and co-workers achieve a deeper insight into molecular desorption by studying the vibrational response of an ensemble of molecules under intense femtosecond laser excitation. Their study motivates the quest for the holy grail of chemistry: the measurement of single-molecule chemistry with simultaneous femtosecond temporal and submolecular spatial scales [10]. A previous observation of a single-molecule vibration suggests that this goal is within reach [11].
This research is published in Physical Review Letters.
References
- A. H. Zewail, “Femtochemistry: Atomic-Scale Dynamics of the Chemical Bond,” J. Phys. Chem. A 104, 5660 (2000).
- K.-i. Inoue, K. Watanabe, T. Sugimoto, Y. Matsumoto, and T. Yasuike, “Disentangling Multidimensional Nonequilibrium Dynamics of Adsorbates: CO Desorption from Cu(100),” Phys. Rev. Lett. 117, 186101 (2016).
- H. Petek, “Photoexcitation of Adsorbates on Metal Surfaces: One-Step or Three-Step,” J. Chem. Phys. 137, 091704 (2012).
- M. Brandbyge, P. Hedegård, T. F. Heinz, J. A. Misewich, and D. M. Newns, “Electronically Driven Adsorbate Excitation Mechanism in Femtosecond-Pulse Laser Desorption,” Phys. Rev. B 52, 6042 (1995).
- R. R. Cavanagh, D. S. King, J. C. Stephenson, and T. F. Heinz, “Dynamics of Nonthermal Reactions: Femtosecond Surface Chemistry,” J. Chem. Phys. 97, 786 (1993).
- H. Petek, “Real-Time Observation of Adsorbate Atom Motion Above a Metal Surface,” Science 288, 1402 (2000).
- M. Dell'Angela et al., “Real-Time Observation of Surface Bond Breaking with an X-ray Laser,” Science 339, 1302 (2013).
- M. Bonn, S. Funk, C. Hess, D. N. Denzler, C. Stampfl, M. Scheffler, M. Wolf, and G. Ertl, “Phonon- Versus Electron-Mediated Desorption and Oxidation of CO on Ru(0001),” Science 285, 1042 (1999).
- K. Ishioka, M. Hase, M. Kitajima, L. Wirtz, A. Rubio, and H. Petek, “Ultrafast Electron-Phonon Decoupling in Graphite,” Phys. Rev. B 77, 121402 (2008).
- H. Petek, “Single-Molecule Femtochemistry: Molecular Imaging at the Space-Time Limit,” ACS Nano 8, 5 (2014).
- S. Yampolsky, D. A. Fishman, S. Dey, E. Hulkko, M. Banik, E. O. Potma, and V. A. Apkarian, “Seeing a Single Molecule Vibrate through Time-Resolved Coherent Anti-Stokes Raman Scattering,” Nature Photon. 8, 650 (2014).