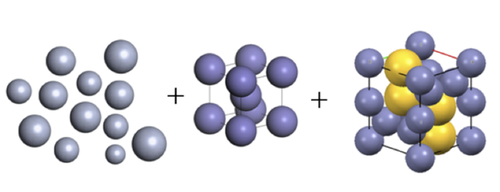
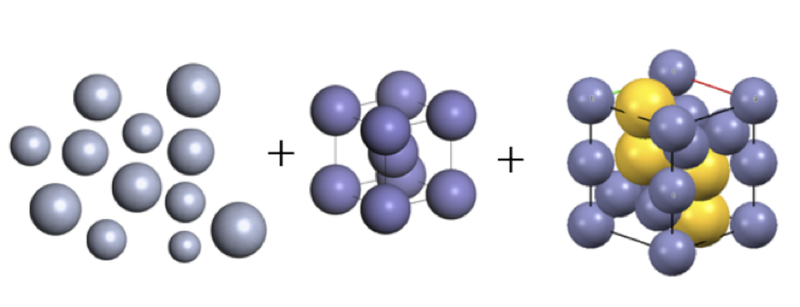
Complex Crystals Form from Heterogeneous Particles
A collection of differently sized balls jostled around in a box is unlikely to spontaneously assemble into a neat pile. But a research team has now demonstrated that spherical nanoparticles suspended in a liquid can pack together in an orderly way, even when the particles have a wide range of sizes. The team was surprised to observe the formation of complex crystals that contained mixed-size particles, an outcome theorists have not looked for when modeling such systems. The researchers say that these results reveal a new route to crystallization, increasing the variety of crystal structures available for industrial purposes.
Particles suspended in liquid can spontaneously crystallize to form a colloidal crystal. These structures serve as model systems that can help researchers understand how real crystals form and can be used to quickly and cheaply make structures such as photonic crystals, which have useful optical properties. If the particles are exactly the same size and interact like bowling balls, the crystallization process is straightforward. Such colloids self-assemble into layered arrays of spheres analogous to crystals made from atoms or molecules. But add some variation to the particle size, and things get messy. For 10-nanometer-diameter particles, a deviation of 0.01 nanometers (nm) is enough to disrupt crystallization.
Theorists have predicted that, in principle, a collection of particles having a variety of sizes could self-assemble if the particles first segregate by size, a process known as fractionation [1]. In this scenario, the particles jostle around until they find exactly the right sized space; multiple crystals form adjacent to one another, each containing nearly uniformly sized particles.
However, the consensus has been that fractionation couldn’t be achieved experimentally. A low concentration of particles would be needed to allow the particles to move long distances and find their similar-sized brethren, but this concentration would be too low for crystals to form at all. Ramp up the concentration, and the dispersion turns into a disordered solid before particles can travel far enough to find their places.
To get around this problem, Lucas Goehring of the Max Planck Institute for Dynamics and Self-Organization in Göttingen, Germany, and his colleagues used charged particles that interact via repulsive electrostatic forces. The particles “sense” each other’s presence and push back on one another long before they physically bump. This repulsion creates empty spaces that give the particles plenty of “wiggle room” to sort quickly into crystals, says Goehring.
Goehring and his colleagues conducted experiments using liquid-dispersed silica particles with diameters ranging from 8 to 24 nm. The distribution of sizes was peaked—the most common diameter was 16 nm, and the number of particles tapered off for diameters both larger and smaller than that. The particles’ repulsive interaction distances were tuned to be a few nanometers greater than their diameters.
The team probed the colloid’s structure using small-angle x-ray scattering. For low particle concentrations, the data suggested a disordered liquid. When the particle volume was increased to 19% of the total volume, the x-ray scattering pattern shifted to that of a body-centered-cubic (bcc) crystal made of roughly 16-nm particles, coexisting with a liquid phase of the remaining particles. At 22% volume fraction, the signature of a crystal structure known as Laves phase appeared. This phase is built from units containing four large particles surrounded by eight smaller ones.
Computer simulations showed that bcc crystals form first from the most abundant particles, in this case approximately 16 nm. The most numerous particle sizes in the leftover larger and smaller particles (in this case around 14 and 18 nm) then start clustering, and a second crystal structure develops from these.
According to the researchers, this crystallization mechanism is extremely robust. “We’ve tried different materials, particles sizes, and interaction strengths,” says Goehring. “Fractionation is fairly common, but the [crystal] phases can differ.”
“This spontaneous formation of such a complex crystal structure, where different lattice sites are occupied by ‘self-selected’ particles of different sizes, has not been seen before” in colloids, says Peter Sollich of Kings College London, a theorist who has studied the effects of size heterogeneity on the behavior of colloids. He says the work opens up the possibility of designing colloidal systems that can self-assemble into specific crystal structures from a generic mixture of particles. “The growing crystallites pick out the particles they need from the mixture and leave the rest behind.” But he says there’s still a lot of work to do, such as predicting the crystal structures that will appear in each case.
This research is published in Physical Review Letters.
–Katherine Wright
Katherine Wright is the Deputy Editor of Physics Magazine.
References
- M. Fasolo and P. Sollich, “Equilibrium Phase Behavior of Polydisperse Hard Spheres,” Phys. Rev. Lett. 91, 068301 (2003).