New Form of Carbon Stores Lots of Gas
Carbon can form diamond, nanotubes, or the nanoscale spheres called buckyballs, as well as several other structures. Now a team has produced what they call carbon honeycomb, a structure that appears to have a huge gas-storage capacity. By slightly altering a common fabrication method, the researchers created what appears to be a 3D honeycomb built from the carbon sheets known as graphene. This structure might be used as a light, energy-efficient fuel storage container for hydrogen fuel cells.
Storing and transporting hydrogen gas efficiently remains a key obstacle to its use as a renewable fuel source. So the US Department of Energy has challenged scientists to develop a system that can store more than 5.5% of its total mass as hydrogen by 2020 [1]. At the moment, storage tanks at very high pressure (for hydrogen gas) or very low temperature (for liquid hydrogen) are the best commercial option, but they require a huge amount of energy to maintain. So many researchers are now focused on developing porous materials that can both trap and release hydrogen gas while consuming much less energy. In theory, carbon nanotubes and other nanostructures, with their very large surface areas, are good candidates, but in practice, access to the gas storage space in these structures is often blocked. Some researchers have proposed a new foam-like carbon structure with a higher gas storage capacity, but it has not yet been demonstrated [2].
To develop better hydrogen storage, Nina Krainyukova of the National Academy of Sciences of Ukraine and Evgeniy Zubarev of the National Technical University, both in Kharkiv, Ukraine, experimented with various ways of making carbon structures. Their most successful technique was similar to the “arc discharge” method, where carbon fragments fly between a pair of charged carbon electrodes and land on a nearby surface to form nanostructures. But instead of two electrodes, the team heated a single carbon rod up to its sublimation point using an electric current. The hot rod produced a vapor containing much smaller carbon fragments than the arc discharge method, according to Krainyukova. These fragments then formed a thin film on a nearby surface. This film proved to be a good gas absorber, as Krainyukova reported in 2009, before she knew its atomic structure [3].
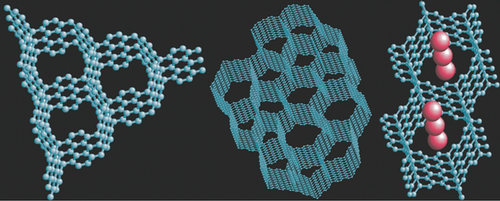
In order to identify the structure, Krainyukova and Zubarev subjected their film to a battery of tests. Electron microscope images revealed a network of hollow channels running perpendicular to the film’s surface, which suggested that the material had a lattice-like structure. From electron diffraction measurements and computer modeling, the researchers determined that the bonds met at 120-degree angles. This angle is a characteristic of graphene sheets and also of sponge-like carbon fragments called schwarzites, but the measured carbon density of the film was too high to be made of schwarzites. Finally, the team again tested the gas capacity of the film, this time with carbon dioxide as well as the krypton and xenon gases tested in 2009, and they found high levels of absorption for each gas. This result eliminated the last of the previously known carbon nanostructures—bundles of nanotubes—which can only bind half as much gas as the new carbon film.
After this last test, Krainyukova says, she and Zubarev had to admit that their carbon film was different from any known structure. They modeled a series of new structures until they found one that matched all of their observations. This winning structure has a repeating pattern of flat graphene sheets bound on edge into hexagons to form a “carbon honeycomb,” as the researchers call it. The open hexagonal channels in the honeycomb are key to its high absorbency, and the team says that the size of these channels could be adapted to fit many different atoms or molecules, including hydrogen gas with an estimated capacity of 8% by mass.
Boris Yakobson of Rice University in Houston, who is also developing carbon-based hydrogen storage, says that this work is a provocative discovery. If confirmed with higher resolution images, the honeycomb structure would be a “remarkable addition” to the current suite of carbon nanostructures, he says. Klavs Hansen of the University of Gothenburg in Sweden agrees. “The obvious application is, after some developments, for gas storage and possibly also molecular sieves.” Farther in the future, he says, there could be still more uses beyond what today’s researchers can imagine.
This research is published in Physical Review Letters.
–Tamela Maciel
Tamela Maciel is a freelance science writer in Leicester, UK.
References
- DOE Hydrogen Storage Targets.
- A. K. Singh, J. Lu, R. S. Aga, and B. I. Yakobson, “Hydrogen Storage Capacity of Carbon-Foams: Grand Canonical Monte Carlo Simulations,” J. Phys. Chem. C 115, 2476 (2011).
- N. V. Krainyukova, “Evidence for High Saturation of Porous Amorphous Carbon Films by Noble Gases,” Low Temp. Phys. 35, 294 (2009).
More Information
The Era of Carbon Allotropes, a 2010 commentary in Nature Materials (with diagrams of carbon structures)