Putting One Foot in Front of the Other
Synthetic molecular motors that move along a track of DNA may prove to be the cargo trains of future nanomachines. But researchers are still struggling to package all the necessary design features of a nanofreight operation into one system. In the 5 December Physical Review Letters, a team reports synthesizing a molecular motor made of DNA that includes three important properties in one molecule. Although still limited in its forward motion, this mechanism could eventually transport cargo in real nanotech devices, according to the team.
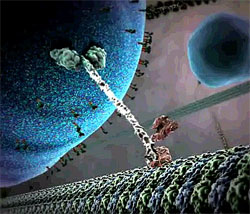
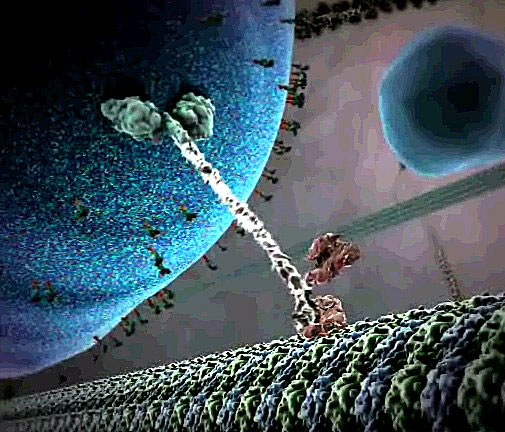
Researchers designing molecular motors for new nanoscale devices have often modeled them after biological motor proteins such as kinesin. Fueled by molecules of ATP, a kinesin molecule “walks” along a biopolymer “track” by alternately attaching and disconnecting each of two “feet,” hauling cellular cargo in its wake. But many of the synthetic motor systems are built from DNA molecules, rather than protein, because the precise interlocking of bases (A, T, G and C) can specify both the structure of the motor and proper foot placement on the track. In addition, a DNA fragment can bind to part of a DNA motor and release energy to fuel forward motion. However, no one has built a two-footed motor with three essential properties: requiring no outside control (autonomy), remaining connected to the track (processivity), and moving in a single direction (directionality).
“What’s critical for achieving all of those things is that you have to coordinate the activities of the two feet,” says Andrew Turberfield of Oxford University in England. He and his colleagues designed a motor with all three characteristics. Imagine a short “connecting rod” of double-stranded DNA parallel to and above a long, single-stranded DNA track. The rod has two identical “legs” of single-stranded DNA hanging down–one at the front and one at the rear–each with a “foot” segment that binds to complementary stretches of DNA sequence in the track. But there isn’t space in the track sequence for both feet to bind completely. So some of the time, the rear “heel” is exposed and accessible to the DNA “fuel” that powers the motor.
To take one step, the rear foot needs to disconnect from the track, flop over the top of the structure, and then reconnect in front. This single step was what the team ultimately demonstrated for its motor. To power the step, they designed two DNA fuel fragments with different sequences that float around in the solution. The first fragment type pries the rear foot off the track and binds to it temporarily, and then the second releases the foot by binding to the first fragment. With the foot freely floating in the solution, it can flop over and bind in front of the other foot. But half of the time, it re-binds to its previous spot on the track, which means no forward progress. Still, the lack of backward motion means that the motor is directional.
The researchers were not able to watch individual motor molecules move. Instead, they tested each stage of the cycle by mixing different combinations of motors, tracks, and fuel fragments together in solution and by controlling the temperature. They then used biochemical analyses to measure the number of DNA complexes of various sizes that appeared under each condition. Each complex represented a different stage in the cycle of taking a step.
Although the researchers have made progress toward a fully autonomous synthetic walker, this motor system is literally just a first step. A next step is to incorporate a power stroke, Turberfield says, a mechanism that would push the motor forward and prevent the free foot from returning to its original place. In addition, Turberfield and his colleagues would like to demonstrate that the motor could move at least 100 nanometers. The system is “ingenious in design,” says John Reif of Duke University. “It is a major step in the emerging field of synthetic molecular robotic devices.”
–Sarah Webb
Sarah Webb is a freelance science writer in Brooklyn, New York.
More Information
3-minute version of The Inner Life of the Cell (low- and high-resolution available), which includes the kinesin segment above.
The Inner Life of the Cell home page, including the 8-minute version.