Movies of Electrons in Atoms
Physicists have long been able to snap atomic-scale pictures by shining a beam of electrons at a target, but filming the electronic structure of an atom as it changes in time is the next goal. A rapid “strobing” of electron pulses less than a millionth of a billionth of a second long should do the trick, according to a theoretical analysis in the 31 December Physical Review Letters. The authors demonstrate with computer simulations that ultrafast electron pulses could track the “breathing” state of an excited atom or the hopping of electrons between atoms in a molecule. Such movies open up the possibility of controlling the electrons that drive chemical reactions.
The electron cloud around an atom or molecule is described by a wavefunction that gives the likelihood of an electron being at each location. This probability distribution is typically fixed, but when energy is added to the system by incoming light or a chemical interaction, the wavefunction starts changing. This electronic rearrangement usually happens in a matter of attoseconds seconds), so researchers need a very fast “flash” to capture the motion. The attosecond light pulses that have recently been produced have wavelengths about 1000 times too large for resolving subatomic features, but electrons have a wave-like nature that can be tuned to the right size. Generating attosecond electron pulses is challenging, in part because electrons repel each other, but several research teams have proposed ways of doing it.
Once attosecond pulses are created, one of the next complications could come from inelastic collisions, in which the electrons around the atom are excited or stripped away by the electron pulse. “You’d rather not change the target that you are measuring,” says Anthony Starace of the University of Nebraska in Lincoln. Previous theoretical work has checked for inelasticity in one-dimensional simulations, but Starace and graduate student Hua-Chieh Shao decided to perform a more thorough investigation in three dimensions. They simulated the scattering of 110-attosecond electron pulses from atomic and molecular targets. To simplify the calculations, each pulse had just a single electron–the pulse duration came from the inherent quantum uncertainty in the time that the electron strikes the target.
Starting with hydrogen, the researchers showed that a carefully timed laser pulse could excite the atom’s one electron from the ground state into a combination (“superposition”) of two separate atomic orbitals, corresponding to the 3p and 4p energy levels. The electron oscillated between the two states with a period of about 6 femtoseconds. Because the 3p orbital is closer to the nucleus than the 4p orbital, the atom appeared to be “breathing”–expanding and contracting. In their simulations, the researchers bombarded a beam of these atoms with electron pulses from one side and recorded the directions of the scattered electrons. The scattering data showed that the atoms periodically became larger targets, and further analysis showed that inelastic scattering only affected a small fraction of the scattering directions.
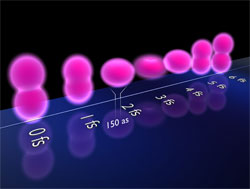
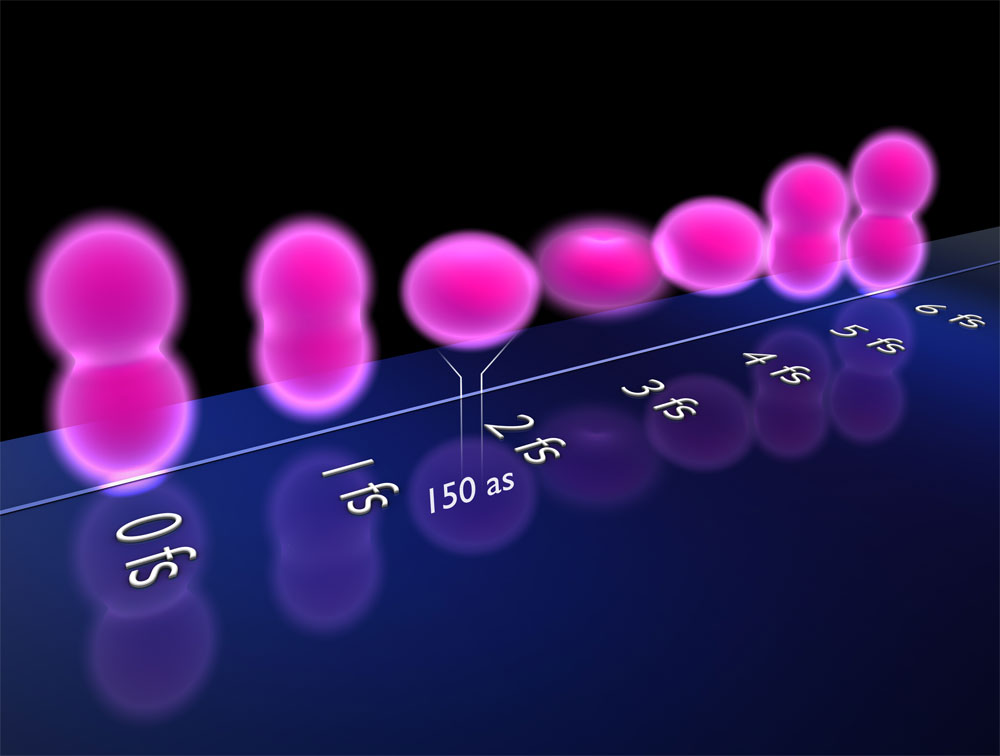
The team’s second case was a molecule composed of two tritium atoms (“heavy” hydrogen). The researchers removed one electron and excited the other so that it periodically switched its orbit about every 4 femtoseconds from one tritium nucleus to the other. The wavefunction looked like two spheres, one surrounding each nucleus, with each sphere alternately growing and then shrinking to nothing. Twice during each cycle the spheres were of equal size, when the electron had equal probability of being around each nucleus. This configuration provided two electron clouds off of which the incoming electron “waves” could scatter, resulting in interference fringes similar to what one sees when light waves go through two slits. This interference pattern remained in the data even when the team added experimental complications, such as variations in molecular alignment and in the separation between nuclei.
“This work is important for experimentalists, because it adds a clear perspective of what could be doable,” says Ernst Fill of Ludwig Maximilians University in Munich. It should help motivate further efforts in developing attosecond electron pulses, he says. “The first effects to be seen experimentally may be those presented in this paper.”
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics Magazine based in Lyon, France.
References
- E. Goulielmakis et al., “Real-Time Observation of Valence Electron Motion,” Nature 466, 739 (2010)