A Better Picture of Molecules
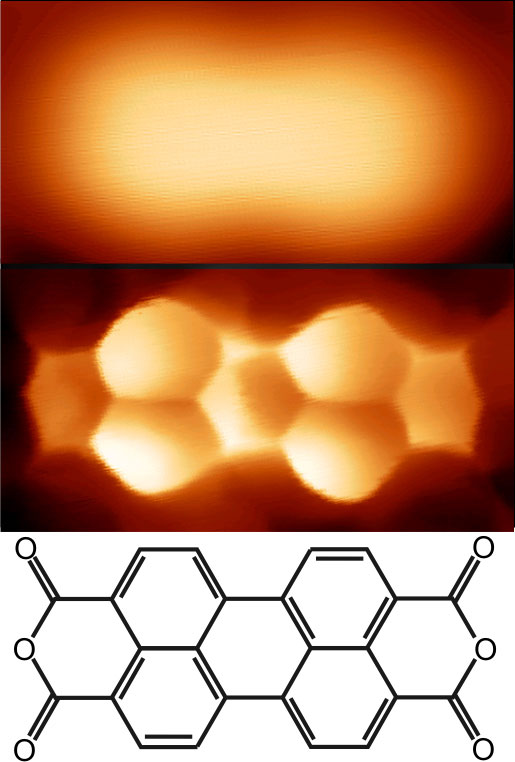
Under a scanning tunneling microscope (STM), molecules usually appear as indistinct blobs. But research published in the 20 August Physical Review Letters shows how a recently developed type of STM can reveal the structure of a molecule in much greater detail. In this type of STM, a single hydrogen or deuterium molecule is attached to the probe tip, and the team showed that the pressure of this molecule against the probe leads to the dramatic improvement in imaging. The technique images the effect of a force on electrical conduction through the probe, which allows a more complete picture of the molecule’s electrons than standard STM provides.
An STM works by bringing a conducting needle, or “tip,” within a nanometer of a surface to be imaged. When a voltage is applied to the tip, electrons flow between the surface and tip, and the conductance through this junction at each point on the surface is measured and used to make an image. The conductance depends on several factors, including a property of the tip and surface called the density of states, which refers to the number of quantum states available to electrons in a given energy range. These states are analogous to the orbitals of electrons in an atom or molecule. The more states, the higher the conductance, and the brighter the image at that location.
In a previous experiment, researchers led by Ruslan Temirov of the Jülich Research Center in Germany discovered they could achieve more detailed images if they covered their STM tip with hydrogen before scanning a thin film of organic (carbon-based) molecules on a metal surface. The resulting images showed bright regions for the atoms, separated by edges that corresponded to the chemical bonds between atoms. But the researchers couldn’t say exactly what this “scanning tunneling hydrogen microscopy” (STHM) was measuring.
To solve that problem, they have now repeated the experiment with deuterium, a heavier isotope of hydrogen, which they happened to have on hand. They scanned an organic molecule called PTCDA resting on a gold surface, which yielded images identical to the previous experiment. The measured conductance values indicated that the image was the product of a single molecule of deuterium attached to the tip, the team reports.
Normally the conductance through an STM junction increases exponentially as the tip approaches the surface. In the team’s experiment, however, the conductance grew relatively slowly and sometimes decreased as the tip approached the surface, over a range of about an ångström. The measurements from this range yielded the high-resolution images.
The researchers explain these effects in terms of the repulsion between electrons associated with the Pauli exclusion principle, which says that two electrons can’t be in the same state and location at the same time. As the tip approaches, the electrons in the deuterium molecule are repelled from the surface, and their orbitals begin to overlap with those of the tip. So the electronic states in the tip rearrange, which changes the conductance through it.
Temirov explains that conventional STM can only probe electronic states in the sample that are within a limited range of energies, because the energy of the detected states depends on the applied voltage. With STHM the conductance also depends on the repulsion generated by Pauli exclusion, which affects states of all energies, so it gives a more complete picture. Other researchers have obtained similar results with atomic force microscopes (AFMs), Temirov says, but STMs are easier to use.
The new work “offers a very convincing and appealing interpretation of the results” of the original experiment, which were “spectacular but very confusing,” says Jan van Ruitenbeek, a nanophysics expert at Leiden University in the Netherlands. “We know now how to go about using it in other experiments and what avenues there are for improving and generalizing it.” Ruitenbeek suggests the technique could be used to identify the products of chemical reactions by imaging them.
–JR Minkel
JR Minkel is a freelance science writer in New York City.