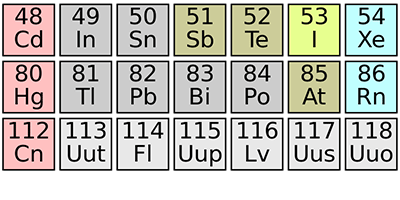
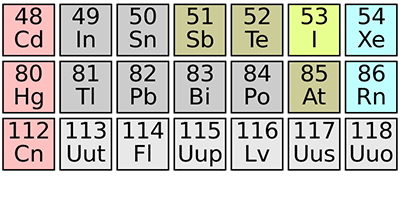
Element 115 Confirmed
Just past copernicium (element ) in the periodic table, several of the few known heavier elements remain nameless. That’s because they’ve only been observed once, and the scientific bodies responsible for naming new elements, the International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics, require, at a minimum, an independent confirmation. That moment has now arrived for element . Dirk Rudolph of Lund University in Sweden and researchers working at the GSI Helmholtz Centre for Heavy Ion Research, Germany, report in Physical Review Letters that they have produced and detected the element, corroborating the first observation in 2003 by scientists in Dubna, Russia.
Most heavy elements can only be synthesized in heavy-ion collisions and have short lifetimes. To produce an element with 115 protons, the researchers at GSI bombarded a rotating target coated with americium ( protons) with a beam of calcium atoms ( protons) traveling at about one-tenth the speed of light. Over the course of three weeks, they observed the radioactive decay of nuclei of element , consistent with what the Dubna scientists had seen. But a new component of the work was the researchers’ ability to detect flashes of emitted light as the nuclei decayed to lighter, more stable isotopes. The energies of these flashes were used to determine the number of protons in several of the daughter nuclei, providing additional support that they originated in element 115.
Rudolph et al.’s experiments aren’t just about naming rights for the new element; their work is part of a larger effort to find new ways to synthesize and study superheavy elements. Some of these elements are expected to lie in a theoretically predicted “island of stability”—a group of isotopes that are relatively stable because of the number of neutrons and protons they contain. – Jessica Thomas