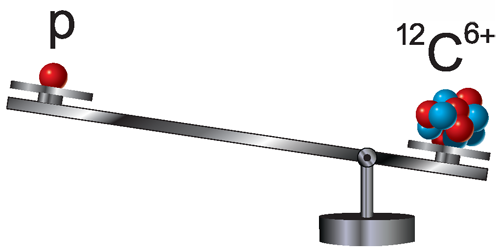
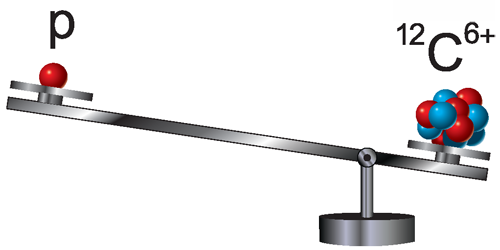
Proton Loses Weight
Knowing the proton mass is crucial for analyzing atomic spectra as well as determining fundamental constants, like the Rydberg constant. A new proton mass measurement by Sven Sturm from the Max Planck Institute for Nuclear Physics, Germany, and colleagues is 3 times more precise than past observations. The team’s value—obtained by comparing a single proton’s motion in a magnetic field to that of a carbon ion—is significantly smaller than the current international-standard estimate.
Precision mass measurements of the proton are typically done with Penning traps, which are combinations of magnetic and electric fields. When placed in such a trap, a proton oscillates back and forth within the electric-field potential well while following a helical path due to the magnetic field. While only the back and forth (axial) motion can be detected directly, the coupling of the different oscillation modes allows researchers to extract the cyclotron frequency with which the proton orbits in the magnetic field. This frequency is proportional to the proton’s charge-to-mass ratio. To obtain the proton mass, this frequency is compared to that of a reference ion, whose mass is known in terms of atomic mass units (defined as 1/12th the mass of the carbon atom).
Sturm and colleagues used ionized carbon ( ) as a reference. To reduce the noise from instabilities of the magnetic and electric fields, the team decreased the time between proton and ion measurements by using separate storage systems for each particle. They also boosted the setup’s sensitivity by including separate motion detectors for the proton and the ion. Their resulting proton mass measurement—with a precision of 32 parts per trillion—disagrees by 3 standard deviations with the CODATA value, which is a compilation of multiple measurements. The team verified their result by performing several cross-checks with different ions.
This research is published in Physical Review Letters.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics based in Lyon, France.