Pinning down the Chemistry of Photosynthetic Water Splitting
Plants efficiently convert sunlight into chemical energy that they store in sugar molecules. Bio-inspired devices performing similar functions could help produce renewable energy. However, scientists don’t yet fully understand the details of the chemical processes involved in photosynthesis. As a consequence, artificial devices can’t yet match the efficiency of naturally occurring photosynthesis. Now, Yulia Pushkar of Purdue University, Indiana, and colleagues have pinned down the steps of a key process in photosynthesis in which water molecules are split into protons, electrons, and oxygen molecules (O 2). Their finding indicates that the chemical bond changes involved in water splitting occur in a different sequence than that predicted by models.
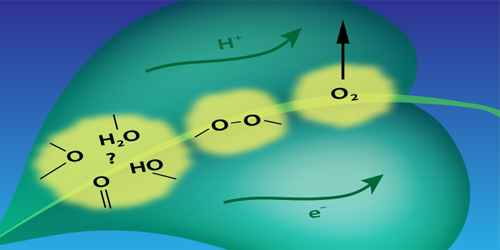
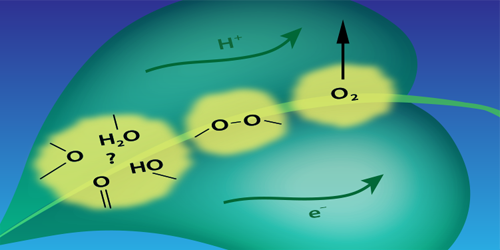
Photosynthetic water splitting is usually described in terms of a chemical reaction driven by a catalyst—a cluster containing manganese and calcium atoms. The reaction involves the sequential absorption of four photons. Each photon strips an electron from the cluster, and each of these ionizations drives the cluster through one in a sequence of four oxidation states that control a series of chemical changes. One of these changes is the formation of an O–O bond that is a necessary prelude to the formation of the O=O bond of O 2. Until now, researchers assumed that the O–O bond formed only after the fourth electron was stripped from the cluster.
Pushkar’s team drove the reaction through its four steps using a sequence of laser pulses. They then monitored the manganese oxidation state after each photon absorption event by measuring the x-ray emission from the cluster. Their analysis of the emission spectra suggests that the O–O bond forms before the fourth electron is removed from the cluster. The researchers suggest that the optimization of the O–O bond formation step may be key to designing better catalysts for energy harvesting applications.
This research is published in Physical Review X.
–Matteo Rini
Matteo Rini is the Deputy Editor of Physics.