How Cells “Measure” Their Own Size
In living things, it’s essential for individual cells to grow and divide in order to create new cells. But how do cells know when they’re big enough to split in two? Their trick seems to be sensing when certain proteins within them have gotten diluted enough, a finding that a team of researchers led by Jan Skotheim of Stanford University, California, planned to share last week at the March Meeting of the American Physical Society. (The meeting was canceled due to concerns about the new coronavirus disease, COVID-19, but Physics is reporting on some of the results that would have been presented.)
The idea that a protein’s dilution could control cell size is a possibility that has only come into view in the last few years, and researchers continue to puzzle out new parts of the process. Skotheim and his colleague Evgeny Zatulovskiy just published a review paper in Trends in Genetics, describing what’s known so far about cell-size control in animals. And thanks to recent experiments, Skotheim’s team may have solved a new piece of the cell-size puzzle, which they will soon report in a paper on the preprint site bioRxiv.org.
Understanding how cells control their size is fundamental to cell biology, Skotheim says. The size of a cell determines how big the organelles within it will be, which in turn affects how the cell produces proteins and other molecules that perform its essential functions. And somehow cells grow and divide in a way that keeps producing cells that are the “right size” for that cell type. For example, a typical human red blood cell is a disk just a few micrometers across, but a skeletal muscle cell is a long, thin structure that can stretch several centimeters.
Skotheim says that he was first drawn to the cell-size puzzle when he saw time-lapse movies of cells growing and dividing as a postdoctoral researcher. By then, Skotheim, whose background is in physics and applied mathematics, had switched to a career in biology. But because of his training, he was especially drawn to understanding how cells can sense their own physical properties and act on that information. “How does something like a cell or microorganism have a mechanism that is able to measure its size?” Skotheim asks. “How is cell size converted to a biochemical signal that triggers cell division?”
Skotheim began looking for molecular mechanisms that might control cell size not long after starting his own lab in 2008. At the time, researchers generally assumed that cells produced more of a given protein as they grew. But Kurt Schmoller, then a postdoc working with Skotheim, wondered if cell size might be tied to the dilution of a protein in the cell. If a cell made a protein only in a fixed amount, the protein would become diluted as the cell enlarged, letting the cell “know” when it was time to divide.
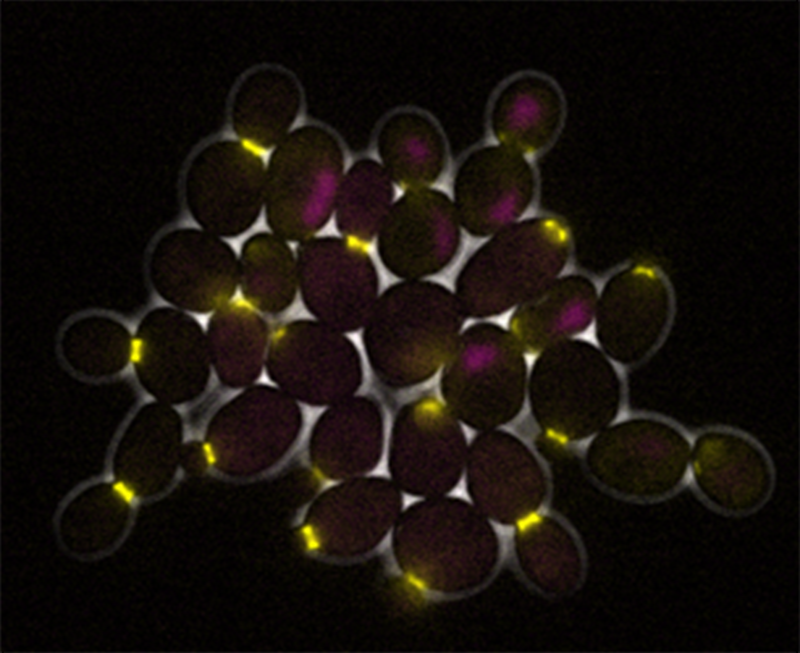
Schmoller, Skotheim, and others measured the concentrations of various proteins in the cytoplasm of growing and dividing yeast cells using time-lapse microscopy. Sure enough, in 2015, they found that a protein called Whi5, which keeps yeast cells from dividing, gets diluted as the cells grow in size. While a cell may produce other proteins continuously as it grows, it only creates a certain amount of Whi5. When the Whi5 protein is diluted to a certain point, the cell divides. Later, as a postdoc working with Skotheim, Zatulovskiy discovered a similar mechanism in human cells. The tumor suppressor protein Rb, known for its role in many cancers, dilutes as cells grow and controls when cells divide in a similar way to Whi5.
Now that the researchers have linked cell size to protein dilution, they want to have a better understanding of the molecular mechanisms that control how these proteins are made. For example, Skotheim’s team recently discovered that yeast cells always create the same amount of Whi5 protein because of a process that’s independent of a cell’s size. The finding, which they plan to describe in the upcoming paper, may help explain why the cells only create a certain amount of the protein rather than produce the protein continuously as they grow.
Skotheim says he thinks that the most important things those with physics backgrounds bring to biology research is a different perspective and a complementary way of approaching questions. While geneticists and microbiologists may focus their efforts on identifying new parts of a cell and understanding the full complexity of a cell, physicists may be more interested in finding what simple concepts—like dilution—underlie a cell’s behavior. “[Physicists] want to boil it down to a concept that can explain as much as possible,” Skotheim says.
–Erika K. Carlson
Erika K. Carlson is a Corresponding Editor for Physics based in Brooklyn, New York.