Measuring Decays with Rock Dating Implications
With a half-life of 1.25 billion years, potassium-40 does not decay often, but its decays have a big impact. As a relatively common isotope (0.012% of all potassium) of a very common metal (2.4% by mass of Earth’s crust), potassium-40 is one of the primary sources of radioactivity we encounter in daily life. Its decays are the primary source of argon-40, which makes up almost 1% of the atmosphere, and the copious amount of heat released from these decays threw off early estimates of the age of Earth made by Lord Kelvin. Potassium-40 is largely responsible for the meager radioactivity in our food (such as bananas), and it is a significant source of noise in some highly sensitive particle physics detectors. This isotope and its decay products are also useful tools in dating rocks and geological processes that go back to the earliest parts of Earth history. And yet some long-standing uncertainty surrounds these well-studied decays. The KDK Collaboration has provided the first direct observation of a rare decay mode of potassium-40 to argon-40 [1, 2]. The measured decay rate implies a smaller probability of this decay mode than previously assumed. The results will have limited but important implications for the field of geochronology, as well as for other fields that either use or seek to avoid the effects of the decay of this ubiquitous element.
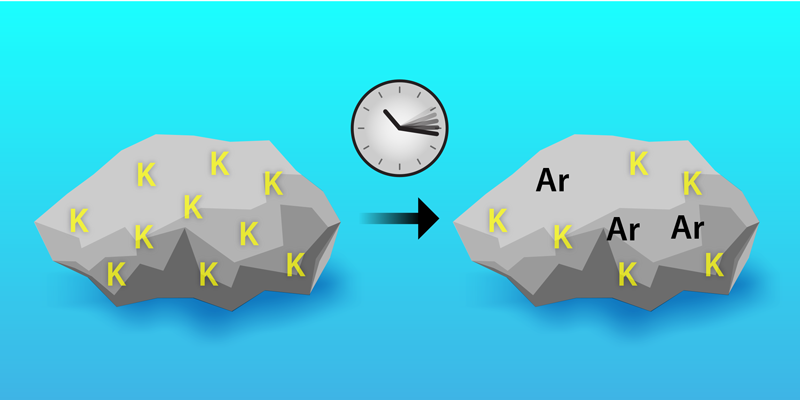
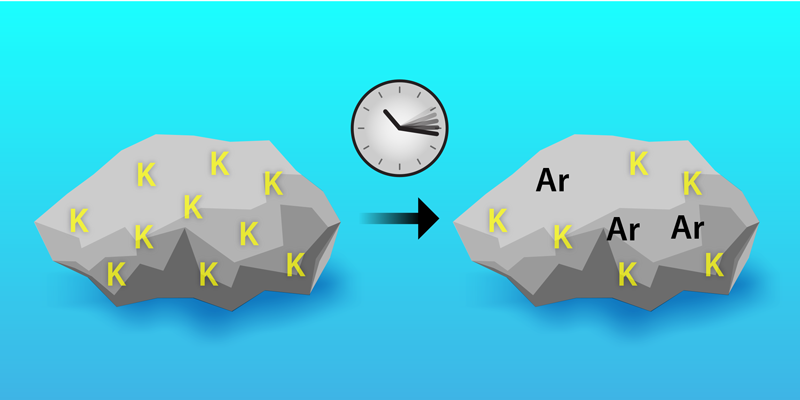
Potassium-40 has a somewhat complicated decay scheme. It’s no uranium, with its chains of long-lived descendants. But it does have some interesting features, with about 90% of potassium-40 decays going to calcium-40 by 𝛽− decay and most of the remaining 10% going to the aforementioned argon-40 by electron capture. When a rock solidifies, it starts off with a certain amount of potassium-40 but almost no argon-40 (Fig. 1). Over time, potassium-40 decays, producing argon-40 that remains trapped in the rock. Geologists can estimate the rock’s age by measuring the concentration of these different elements. One way to do this—so-called potassium–argon dating—is to measure the total potassium (mostly potassium-39) and calculate the amount of potassium-40 from the known relative abundances. This value is then combined with an argon-40 measurement to calculate an age.
An alternative dating method—more commonly used nowadays—is to transmute a small amount of the potassium-39 in a rock into argon-39. This argon-39 acts as a proxy for the amount of potassium and by extension for the amount of potassium-40. Geologists can therefore use the ratio of argon-39 to argon-40 to determine the age of the rock. This argon–argon dating technique offers the advantage that the mass spectrometry measurements target isotopes of the same element, which can be done more quickly and precisely than comparisons of different elements. The potassium–argon transmutation occurs through neutron activation in a reactor, a somewhat messy process that imparts a slew of additional reactions and corrections on the age determination.
To convert argon and potassium abundances from both methods into an age, one must quantify the overall decay rate of potassium-40, as well as the relative decay rates to each descendant (branching ratios). This can be surprisingly difficult, as it requires accurately measuring both the parent isotope and a sufficient number of extremely rare decays. The work by the KDK Collaboration deals with a rare subset of the approximately 10% of the potassium-40 that decays to argon-40 by electron capture. About 99% of this 10% goes to an excited state of argon-40, which is a useful feature because the subsequent (nearly immediate) decay to the ground state of argon-40 emits a characteristic gamma ray. Researchers can measure that gamma ray to help quantify the rate of this process and also to correct for its presence in other situations, such as in dark matter observatories where radioactive decays are a significant interference.
However, a very small subset of electron-capture decays of potassium-40 go directly to the ground state of argon-40, meaning there is no gamma ray, just low-energy x-rays that are difficult to isolate. The result of each electron capture is the same as far as geochronology is concerned—both decays produce a stable argon-40 nucleus—but the rate of the direct-to-ground-state subset is much harder to measure. Long predicted, it has been estimated to be as much as 2% of the decays to argon-40 [3, 4] but has been omitted entirely from some commonly used decay models [5]. The KDK work, using careful measurement of the x-ray and gamma-ray spectra produced by an enriched potassium source (described in [1] and in more detail in [2]), shows it is in fact closer to half that value. This result represents the first direct measurement of the decay rate of potassium-40 to the argon-40 ground state, and it also implies a need for a redetermination of other related decay rates. As a consequence, some potassium–argon ages may require corrections of close to 1%, affecting the age of some old meteorites and rocks by tens of millions of years.
The immediate implications for argon–argon dating, as pointed out by the researchers, will be limited. The reason for this is that argon–argon dating is a relative technique; standards of known age are placed in the nuclear reactor along with the rock samples so that the same proportion of potassium-39 is transmuted to argon-39 in both. One of the advantages of this approach is that the uncertainties in many physical constants—such as the decay rates—partially cancel out because they apply to the age-determining factors of both the standards and the coirradiated samples. The absolute ages of many common standards will also not be affected, because for the most part they are based on other chronometers—employing either other decay schemes (primarily uranium–lead [6]) or techniques such as calibrating multiple dated layers in a sedimentary sequence using astronomical cycles [7]. This, however, is a weakness of the argon–argon method, as it pins all dates to the systematic biases inherent to these other methods. A medium-term goal for the field is to improve direct calibration of the argon–argon method using potassium–argon dating to the point that this calibration can be used for independent comparison with techniques like uranium–lead ones. This will require accurate and precise accounting of all physical constants involved in the decay of potassium-40 to argon-40 and its incorporation into minerals, including rare decay modes that affect the overall decay constant and branching ratio of potassium-40. As progress in the field of high-precision geochronology continues, corrections like the one considered here will only grow in importance.
References
- M. Stukel et al. (KDK Collaboration), “Rare 40K decay with implications for fundamental physics and geochronology,” Phys. Rev. Lett. 131, 052503 (2023).
- L. Hariasz et al. (KDK Collaboration), “Evidence for ground-state electron capture of 40K,” Phys. Rev. C 108, 014327 (2023).
- D. W. Engelkemeir et al., “Positron emission in the decay of K40,” Phys. Rev. 126, 1818 (1962).
- J. Carter et al., “Production of 40Ar by an overlooked mode of 40K decay with implications for K-Ar geochronology,” Geochronology 2, 355 (2020).
- K. Min et al., “A test for systematic errors in 40Ar/39Ar geochronology through comparison with U/Pb analysis of a 1.1-Ga rhyolite,” Geochim. Cosmochim. Acta 64, 73 (2000).
- P. R. Renne et al., “Joint determination of 40K decay constants and 40Ar∗/40K for the Fish Canyon sanidine standard, and improved accuracy for 40Ar/39K geochronology,” Geochim. Cosmochim. Acta 74, 5349 (2010).
- K. F. Kuiper et al., “Synchronizing rock clocks of Earth history,” Science 320, 500 (2008).