A Cleaner Route to Steel Production
Using hydrogen as a reactant to produce steel is potentially more environmentally friendly than using carbon, as in the conventional process. But a number of challenges must be overcome before this switch can be made on an industrial scale. One problem is that one of the reactions needed to make steel using hydrogen is mysteriously sluggish. Xuyang Zhou and his colleagues at the Max Planck Institute for Iron Research in Germany have now identified the main cause of the slowdown and have suggested a method to mitigate it [1].
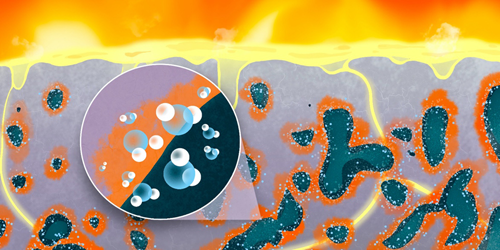
Steel is made through a redox (electron-trading) reaction in which iron oxide reacts with another material to produce steel and an oxide by-product—carbon dioxide if the reactant is carbon, water if it’s hydrogen. Zhou and his colleagues knew that the removal of oxygen atoms during this process leaves behind nanometer-to-micrometer-scale pores in the iron oxide. Through simulations and electron-microscopy observations, they discovered that, when hydrogen is the reactant, water trapped in these pores could reverse the reduction (electron-adding) process required to produce steel and actually reoxidize (remove electrons from) the iron, slowing the overall reaction.
The team offers a solution to mitigate this slowdown effect. If the pores were sufficiently interconnected to form channels, then the water would have a chance to percolate out of the material before reoxidizing it. Zhou says that the team should be able to achieve the required pore morphology by controlling the temperature, pressure, and other parameters during the reaction.
Steel production currently accounts for 7–9% of global carbon dioxide emissions, says Zhou, so finding an alternative method is essential to reducing greenhouse gases and addressing climate change.
–Katie McCormick
Katie McCormick is a freelance science writer based in Sacramento, California.
References
- X. Zhou et al., “Effect of pore formation on redox-driven phase transformation,” Phys. Rev. Lett. 130, 168001 (2023).