Confined liquid controversies near closure?
Water excites intense controversy among researchers. Its crucial role in the natural and biological world is unquestioned but much of the agreement stops [1] when it comes to details. In particular, how water behaves at interfaces is a central mystery. Some interfaces are hydrophobic [2], but here we are interested in polar surfaces, which attract water. This situation is common in the natural world, from earth and rock formations to buildings and highways, and also in the biological world, especially in living cells. When water is restricted to a thin layer at the molecular level, one refers to it as being confined. One of the most baffling aspects about confined water is whether it behaves in a special, even unique, manner or similarly to other confined fluids.
Now, a pleasing resolution of earlier contradictory findings is presented in Physical Review Letters by Shah Khan, George Matei, and Peter Hoffmann of Wayne State University in the US, writing with Shivprasad Patil of the Indian Institute of Science Education and Research, Pune, India [3]. In an experimental tour de force, they use atomic force microscopy (AFM) to make precise measurements of phase during sinusoidal oscillations of the film thickness with an amplitude that is less than the diameter of a water molecule, giving them access to the important linear-response region, which was inaccessible to most prior measurements. Their main result is that, provided that the confined film is formed at a rate that exceeds some critical value, water confined between the oscillating AFM tips and a single crystal (mica) shows progressively more sluggish mechanical relaxation as the film thickness decreases below – diameters of the water molecule. At first, viscous losses at a fixed frequency rise together with the stored elastic energy, but when the thickness is sufficiently small, the viscous losses pass through a maximum and subsequently decrease. It is worth noting that this behavior is characteristic of supercooled fluids when temperature is lowered or pressure is raised. This study puts into perspective earlier studies that seemed to contradict one another. Researchers who concluded that confined water has an effective viscosity nearly the same as bulk water [4–6] and those who concluded otherwise [7–11] are now shown by Khan et al. to have been operating in different regimes of experimental parameters—the quench rate and film thickness.
In a similar spirit, also noteworthy in its resolution of earlier contradictory findings regarding confined fluids, is a study in Physical Review Letters [12] in which Lionel Bureau of the CNRS-Université Paris , France, studied an entirely different fluid, in this case a nonpolar fluid of compact shape, and reported a progressive dynamic slowdown upon increasing confinement between parallel single crystals (mica). This study is important for two reasons. First, these trends, similar to those for the aqueous system studied by Khan et al. [3], are lovely to notice, as this suggests that the behavior may be generic, in spite of the fact that water has a lower melting temperature and its structure is dominated by hydrogen bonding. Second, this study also goes a long way towards settling controversies that had lingered in the literature for too long. Earlier, this nonpolar fluid had been reported to shear-melt when confined to a thin enough layer [13,14], or to display an effective viscosity more or less unchanged from the bulk fluid [15,16], or to display behavior akin to supercooled fluids, progressively more so with diminishing film thickness [17,18]. In his paper, Bureau [12] proposes explanations for these seeming inconsistencies. As happens so commonly when scientists disagree, the problem lies not in the measurements but in how they are interpreted.
These two studies show that confined fluids are neither like bulk fluids nor like bulk crystalline solids. They appear to be an intermediate kind of matter whose finite size and surface-fluid interactions impart unique structural, thermodynamic, and dynamic properties. While their inherently heterogeneous character and sluggish relaxation times are reminiscent of supercooled fluids, it would be valuable to test this with a wider array of experiments. It is not yet feasible to perform nanocalorimetric experiments, for example, but they would be important to explore the limits of the analogy.
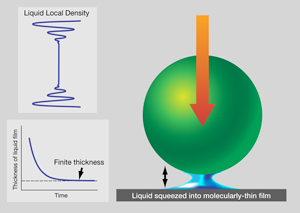
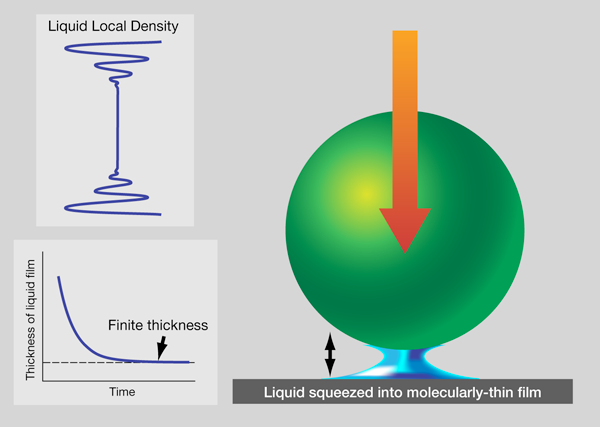
For now, it is worthwhile to ask how one might expect to form a confined fluid. Consider the following thought experiment. Let a ball fall onto a table with a layer of liquid on it, as shown in Fig. 1. Of course the liquid squirts out. How the rate of squirt slows, as the liquid thickness becomes less than the diameter of the ball, was solved at the continuum level in the th century. Twentieth century scientists, attuned to explanation on the molecular level, discovered that the liquid thickness stabilizes at a few molecular diameters: the liquid supporting the weight of the ball [19]. While skeptics might seek to pick holes—such as by invoking electrostatic repulsion between the ball and table—in the example shown in Fig. 1, the general picture is valid. Intuitively, we can say that confinement-perturbed mechanical responses reflect confinement-induced changes in the local packing of the molecules and their mobility.
Thanks to these recent studies by Khan et al. [3] and Bureau [12], experimental controversies regarding dynamic mechanical properties of confined fluids between solid slabs appear to be nearing resolution. Much remains to be done in the way of definitive explanation. It is natural to think of explaining these strikingly altered mechanical properties in terms of how short-range packing of molecules is modified by confinement, this in turn being modified by commensurability or incommensurability between the length scale of film thickness relative to the molecular size. While analogies to glasses and supercooled fluids [11,17] are appealing, such explanations remain qualitative. The problem is that when it comes to confined liquid physics, high density, short-range packing, and dynamical rearrangements of structure are quite interrelated.
We conclude by expressing some challenges for future scientists and engineers. First, the challenge of techniques: Experimentalists do not yet have a clear enough picture of how to augment mechanical measurements, highlighted in this Viewpoint, in the quest for more direct information in terms of structure factor and coefficients of molecular transport, but theorists will need more than such measurements to test and extend their models. Distinguishing between model-dependent and model-independent analyses of mechanical experiments is another challenge, as Khan et al. point out [3]. Yet another challenge is to expand the frequency range, which presently is narrow [3]. Theorists and computer simulators also face deep challenges: as fully quantum simulations are unrealistic owing to the large number of molecules involved, how does one ensure the robustness of conclusions relative to other reasonable choices of potentials, not only those for the liquid-liquid interactions but also those for surfaces with which the liquids interact? The more deeply this physical situation is understood, the more effectively physics can contribute to alleviating the many problems of society that hinge upon controlling the behavior of water and other confined fluids.
How to go beyond these model studies [3,12] in which the tip-surface separation is so exquisitely well defined? There are huge classes of confined fluids that inherently do not meet this condition, either because the confining surfaces are just about as deformable as the intervening fluid, or because the surface-surface separation differs rapidly from spot to spot, or combinations of these. Prominent examples are biomolecules, which certainly also involve confined water. To meet this need, it will probably be necessary to develop new kinds of spatially resolved methods. AFM experiments that address this sort of question are extremely challenging, though they have begun to be attempted using sharper tips that allow spot-to-spot measurements on rough surfaces [20].
This interdisciplinary problem of confined fluids becomes increasingly topical as direct contact is made with water in biological systems. There are issues of structural water molecules of relatively low mobility that may function to stabilize protein dynamics and structure; of water that is transported single-file through ion channels; and also of water in hydration shells, which is less structured but still different from that of bulk water. The notion of “just water” is an idealization. Ions are always present, and always play key roles in the interaction of water with surfaces. For experimentalists, much work remains to be done before a clean separation can be made between the action of “confined water” [3] and additional contributions from charged ions embedded within it.
References
- P. Ball, Life’s Matrix: A Biography of Water (Farrar, Straus and Giroux, New York, 2001)[Amazon][WorldCat]
- S. Granick and S. C. Bae, Science 322, 1477 (2008)
- S. H. Khan, G. Matei, S. Patil, and P. M. Hoffmann, Phys. Rev. Lett. 105, 106101 (2010)
- U. Raviv, S. Perkin, P. Laurat and J. Klein, Langmuir 20, 5322 (2004)
- U. Raviv, P. Laurat, and J. Klein, Nature 413, 51 (2001)
- U. Raviv and J. Klein, Science 297, 1540 (2002)
- M. Antognozzi, A. D. L. Humphris, and M. J. Miles, Appl. Phys. Lett. 78, 300 (2001)
- S. Jeffery, P. M. Hoffmann, J. B. Pethica, C. Ramanujan, H. O. Ozer, and A. Oral, Phys. Rev. B 70, 054114 (2004)
- T. D. Li, J. P. Gao, R. Szoszkiewicz, U. Landman, and E. Riedo, Phys. Rev. B 75, 115415 (2007)
- R. C. Major, J. E. Houston, M. J. McGrath, J. I. Siepmann, and X. Y. Zhu, Phys. Rev. Lett. 96, 177803 (2006)
- Y. Zhu and S. Granick, Phys. Rev. Lett. 87, 096104 (2001)
- L. Bureau, Phys. Rev. Lett. 104, 218302 (2010)
- E. Kumacheva and J. Klein, J. Chem. Phys. 108, 7010 (1998)
- H. Yoshizawa and J. N. Israelachvili, J. Phys. Chem. 97, 11300 (1993)
- T. Becker and F. Mugele, Phys. Rev. Lett. 91, 166104 (2003)
- A. Maali et al., Phys. Rev. Lett. 96, 086105 (2006)
- A. L. Demirel and S. Granick, Phys. Rev. Lett. 77, 2261 (1996)
- Y. Zhu and S. Granick, Langmuir 19, 8148 (2003)
- J. N. Israelachvili, Intermolecular and Surface Forces (Academic Press, London, 1992)[Amazon][WorldCat]
- T. Uchihashi, M. Higgins, Y. Nakayama, J. E. Sader, and S. P. Jarvis, Nanotechnology 16, S49 (2005)