Chemical Synthesis in Small Spaces
Can miniscule reaction chambers such as atmospheric aerosol droplets [1], prebiotic aerosols [2], or microscale compartments discovered at hydrothermal vents [3] enhance chemical synthesis? A variety of natural processes rely on chemical synthesis within mesoscale confinements, including those that may have resulted in primitive life forms arising from a collection of nonliving molecules [2–4]. It is known, however, that entropic contributions thermodynamically disfavor synthesis reactions; for example, formation of one product molecule from two reactant molecules results in a high loss of mobility and thereby a decrease in entropy [5]. A report in Physical Review Letters by Ali Fallah-Araghi at the University of Strasbourg, France, and colleagues [6] describes a universal mechanism that could help overcome the thermodynamic unfavorability of these reactions. Specifically, they argue that these synthesis reactions are more favorable if they take place in a microcompartment than if they occur without confinement.
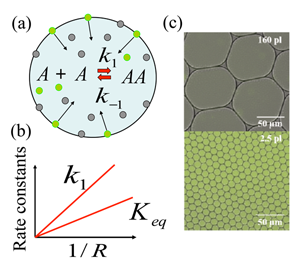
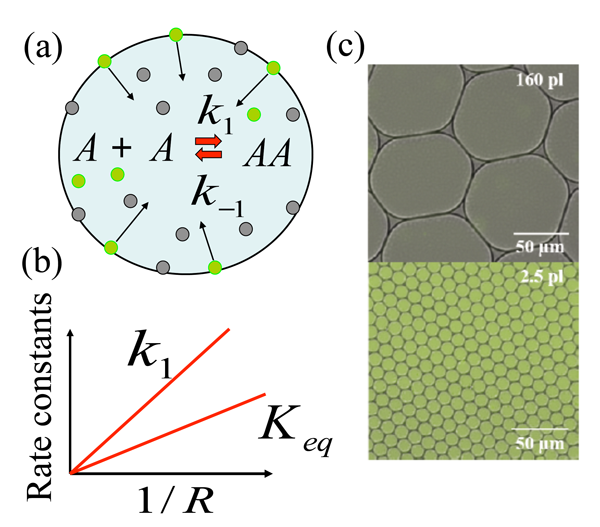
The research team focused on a model chemical reaction where two nonfluorescent molecules A (amine and aldehyde, which for simplicity are considered the same [6]) react to form a fluorescent product AA (imine), and found that the chemical synthesis is strongly enhanced if the reaction took place in micrometer-sized droplets. They explain this enhancement through a mechanism in which reactants are adsorbed to the droplet surface, and then react, desorb, and diffuse back into the interior of the droplet. This model chemical reaction, A+Ak1⇔k-1AA, takes place within the aqueous droplet [Fig. 1(a)], with k1 and k-1 marking the forward and backward reaction rate constants, respectively. The authors’ choice of model reaction with nonfluorescent reactants A and fluorescent product AA allowed them to readily visualize any enhancement of chemical reaction using fluorescence imaging. The droplets are dispersed in an oil phase and are stabilized by the nonionic surfactant; such droplets provide ideal soft microcompartments for the model chemical reaction. Fallah-Araghi et al. used monodispersed droplets with radii from 8 to 34 micrometers, with which they demonstrated the profound effect of confinement on this reaction. Both the apparent forward reaction rate constant k1 and the apparent equilibrium constant Keq=k1/k-1 were found to be inversely proportional to the droplet radius R [Fig. 1(b)]. As can be seen from Fig. 1(c), the enhanced synthesis in smaller droplets (lower image) results in clearly visible color changes, which correspond to the higher concentration of fluorescent product AA, with respect to larger droplets (upper image).
To explain their experimental findings, the authors develop a minimal model that accounts for the following four reversible processes: (i) Reaction A+A⇔AA occurring within the droplet’s interior; (ii) the same reaction taking place at the droplet’s inner surface; (iii) binding of the reactants to the droplets surface; and (iv) dissociation of the species from the droplet surface back into its interior.
If the species A and AA were nonreactive and only processes (iii) and (iv) took place, the equilibrium would represent a state in which the rates of adsorption of each of these species onto the surface were exactly balanced by their rates of desorption back into the bulk. In this case, the bulk and surface densities would assume unsaturated values defined by the ratios of bulk-to-surface adsorption and surface-to-bulk desorption rate constants. (Because the affinities of the species to the surface are relatively low the surface is unsaturated so that there is always a portion of the surface available for the reactants to adsorb.)
In the system of interest here, however, along with adsorption and desorption processes (iii) and (iv), reversible reactions in the bulk [process (i) above] and on the inner surface of the droplet [process (ii)] take place. Adsorption and desorption induces gradients of species close to the droplet’s surface thereby modifying the course of chemical reaction. The affinity between the product and the surface is relatively low, so there is no accumulation of AA observed at the surface. Namely, the product AA generated at the surface [process (ii)] desorbs into the immediate vicinity of the surface within a characteristic length scale ξ , which is defined by the diffusion coefficient and the characteristic decay time of the product AA within the bulk.
This is where confinement (that is, the size of the droplet, R) comes into play. For large droplets with R>>ξ the adsorption-desorption processes modify the reaction only in the immediate vicinity of the droplets’ surface. On the other hand, in the limit of small droplets, where condition R<<ξ holds, the product AA is redistributed within the entire droplet, as can be clearly seen from the fluorescent images for the smaller droplets in the lower image in Fig. 1(c). An analytical solution of the model in this limit gives the apparent equilibrium constant as Keq∼1/R, which agrees well with experimental observations. The magnitude of enhancement of the chemical reaction by this reaction-adsorption mechanism is strikingly large: the authors report a 45-fold increase in the forward reaction rate constant k1 for the smallest droplets considered in their experiments.
The mechanism for improving thermodynamically unfavorable synthetic reactions suggested by Fallah-Araghi et al. is noncatalytic and universal in the sense that it does not require any size or shape complementarity; furthermore, it requires only relatively low binding energies of the reactants to the surface. Such universality of the proposed mechanism makes it relevant to a variety of other chemical processes occurring within microscale confinement. The proposed mechanism could potentially allow one to explain enhanced chemical syntheses during “prevolutionary” dynamics [7]. It could also help understand the underlying reason for the high concentration of organic molecules in present-day secondary (that is, formed in the atmosphere) aerosol particles. These are the majority of aerosol particles in high-population areas that play an important role in many aspects of everyday life, from visibility reduction (haze) to health impacts of air pollution to acid and nutrient deposition to ecosystems [1].
Finally, this new work on controlling chemical reactions within microscale compartments could pave the way for engineering novel synthetic structured materials. Prior research shows that nonlinear chemical dynamics combined with compartmentalization and well-defined connections between the compartments allow us to control the evolution of complex biomimetic oscillatory systems [8]. The mechanism suggested by Fallah-Araghi et al. provides an ideal playground for designing new types of biomimetic structural materials. By controlling the size of the confinement, the specific chemistry of the reaction, and the affinity of the reactants to the droplets’ inner surface, one can precisely regulate the synthesis of the product and thereby control properties and functionality of such synthetic structural media.
References
- C. E. Kolb and D. R. Worsnop, “Chemistry and Composition of Atmospheric Aerosol Particles,” Ann. Rev. Phys. Chem. 63, 471 (2012)
- C. M. Dobson, G. B. Ellison, A. F. Tuck, and V. Vaida, “Atmospheric Aerosols as Prebiotic Chemical Reactors,” Proc. Natl. Acad. Sci. U.S.A. 97, 11864 (2000)
- E. V. Koonin and W. Martin, ”On the Origin of Genomes, and Cells within Inorganic Compartments,” Trends Genet. 21, 647 (2005)
- K. Ruiz-Mirazo, C. Briones, and A. de la Escosura, “Prebiotic Systems Chemistry: New Perspectives for the Origins of Life,” Chem. Rev. 114, 285 (2013)
- M. I. Page and W. P. Jencks, “Entropic Contributions to Rate Accelerations in Enzymic and Intramolecular Reactions and Chelate Effect,” Proc. Natl. Acad. Sci. U.S.A. 68, 1678 (1971)
- Ali Fallah-Araghi, Kamel Meguellati, Jean-Christophe Baret, Abdeslam El Harrak, Thomas Mangeat, Martin Karplus, Sylvain Ladame, Carlos M. Marques, and Andrew D. Griffiths, “Enhanced Chemical Synthesis at Soft Interfaces: A Universal Reaction-Adsorption Mechanism in Microcompartments,” Phys. Rev. Lett. 112, 028301 (2014)
- M. A. Nowak and H. Ohtsuki, “Prevolutionary Dynamics and the Origin of Evolution,” Proc. Natl. Acad. Sci. U.S.A. 105, 14924 (2008)
- I. R. Epstein, V. K. Vanag, A. C. Balazs, O. Kuksenok, P. Dayal, and A. Bhattacharya, “Chemical Oscillators in Structured Media,” Acc. Chem. Res. 45, 2160 (2012)