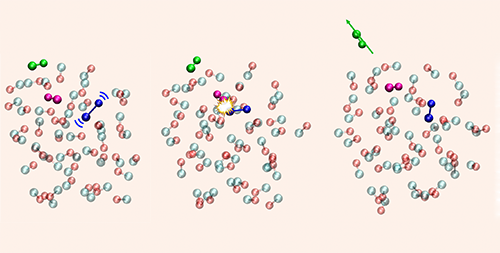
Carbon Monoxide Leaves Cosmic Ice with a Kick
In cold, star- and planet-forming regions of the cosmos, gaseous carbon monoxide (CO) can freeze on the surfaces of dust grains. Ultraviolet (UV) light can cause CO ice to transform back into a gas, counterbalancing the freezing process. Researchers have previously studied such photo-induced desorption in the lab, but struggled to develop a microscopic theory able to reproduce observations. Now a team of theorists at the University of Lille and experimentalists at Sorbonne University, both in France, has proposed such a theory [1]. A better understanding of this process could aid exoplanet-chemistry studies, as a CO molecule is the starting point for the synthesis of many organic compounds.
To perform controlled CO-desorption experiments, the Sorbonne University team exposed CO ices to UV light in ultrahigh vacuum. Through spectroscopy, they watched the desorption process while measuring the populations of rotational and vibrational states of the involved CO molecules.
Molecular dynamics simulations by the University of Lille team suggests the desorption process can occur in three steps. First, UV light excites a CO molecule in the ice, causing it to vibrate. Second, this vibrating molecule “kicks” one or two neighboring molecules, transferring its vibrational energy to them. Third, a cascade of molecular interactions transfers energy from these vibrating molecules to other CO molecules at the surface, giving them sufficient energy to overcome the binding energies and leave the ice.
The researchers say that the simulation predictions offer “remarkable” agreement with their experiments and with all “other experimental findings of the past decades.” They add that the three-step mechanism could play a role in the photo-desorption of more complex ice mixtures in astrophysical settings and in Earth’s atmosphere.
–Matteo Rini
Matteo Rini is the Editor of Physics Magazine.
References
- S. Del Fré et al., “Mechanism of ultraviolet-induced CO desorption from CO ice: Role of vibrational relaxation highlighted,” Phys. Rev. Lett. 131, 238001 (2023).