Synchronized Cell Motion without Fluid Interactions
When the last common ancestor of plants and animals populated the Earth more than 1.5 billion years ago, it already possessed whip-like organelles known as cilia and flagella. Nowadays, these slender ~10-micrometer-long appendages are common to a large number of species—including humans—where they have evolved to participate in a disparate variety of tasks like mechanochemical sensing, cell cycle regulation, and vision [1].
The most immediately striking property of cilia and flagella, however, is their unrelenting motion. Beating at typical frequencies of a few tens of hertz, these active filaments propel spermatozoa towards the egg, break left-right symmetry in developing mammalian embryos, and clear pathogens off human airways, to cite a few of their essential functions. Motile cilia and flagella show a peculiar and nearly universal tendency to synchronize and produce large-scale patterns of coordinated beating [2]. Previous work has found that fluid-mediated stresses can coordinate flagella belonging to different cells [3], but new experiments suggest the situation is different for flagella belonging to the same cell [4]. Studying unicellular algae in a periodically driven flow, Greta Quaranta from the Delft University of Technology, Netherlands, and her colleagues have shown that hydrodynamic forces are not enough to synchronize the two flagella of a common single-cell alga. The researchers identify a specific fiber within the cell as a possible source of direct mechanical connection leading to the observed synchronization. This may provide new insights into mechanical coupling and force transmission within a single cell.
The synchronized undulations commonly found within groups of flagella and cilia are similar to Mexican waves in a football stadium. These so-called metachronal waves are thought to increase the propulsion efficiency of the group [5], thereby providing a competitive edge in the fight for survival. But knowing the benefits of synchronization doesn’t tell us how it emerged in the first place. Flagella and cilia interact with each other through the hydrodynamic flows that they generate, and this coupling can lead to synchronization, as recently confirmed in a study of different cells extracted from the multicellular flagellated green alga Volvox carteri [3]. However, there is likely more than one mechanism for synchronization, as Quaranta and her colleagues have now shown [4].
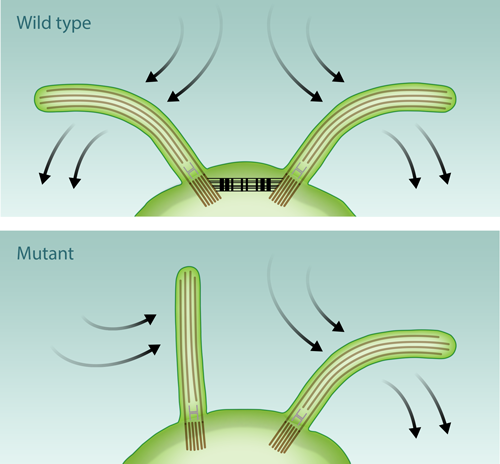
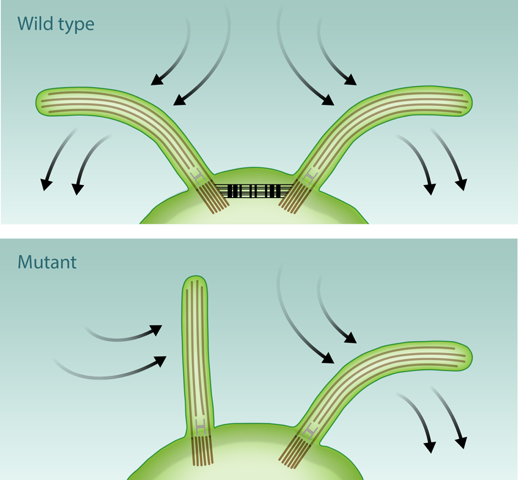
In their study, the researchers worked with the unicellular microalga Chlamydomonas reinhardtii, a common model system for flagellar physiology. These organisms have a pair of flagella that move together in a breaststroke-like motion (see Fig. 1). The authors captured individual Chlamydomonas cells with a micropipette inside a small flow chamber in which the surrounding fluid was pushed back and forth in a repeated cycle. A camera recorded the motion of the isolated swimmer, detailing the response of its two flagella to the externally imposed periodic background flow. The experimentally observed phase dynamics of these forced oscillators is in excellent quantitative agreement with the predictions of a simple model, the stochastic Adler equation. This widely used equation describes weakly interacting self-sustained phase oscillators, as found in dynamical systems ranging from populations of neurons to coupled Josephson junctions [6].
In previous studies of flagellar motion [7], researchers have used the stochastic Adler equation to estimate the effective interflagellar coupling strength , but the model, by itself, does not specify the origin of the coupling. With their experimental setup, Quaranta et al. were able, for the first time, to directly measure the contribution of fluid forces to .
Varying independently the frequency and amplitude of the imposed flow and analyzing the distribution of phase differences between the flagella and the external forcing, the authors evaluated directly how depends on the flow speed. The results showed that the flagella moved in phase with the external flow if the driving frequency was within 5% of the natural frequency of the flagella (roughly 54 hertz). However, for larger frequency differences, the team found that the flow could not entrain the flagella even with the large hydrodynamic forces obtained by increasing the flow speed to 10 times the natural swimming speed of the algae. The implication is that Chlamydomonas has a weak hydrodynamic coupling between its flagella, that is only able to induce synchronization for small frequency differences (less than 0.5%) under normal swimming conditions. However, this would appear to contradict measurements of intrinsic frequencies, which are the rates at which single flagellum beat in isolation. In Chlamydomonas, the two flagella can have intrinsic frequencies that differ by as much as 30%, and yet the flagella still synchronize. Since hydrodynamic interactions are not enough to drive that coordination, Chlamydomonas must rely on intracellular coupling.
An appealing possibility is that of mechanical coupling through calcium-sensitive contractile fibers that join the flagellar bases within the cell (see Fig. 1). This was already suggested as a possibility more than forty years ago but never tested. Quaranta and collaborators investigated for the first time the flagellar synchronization in a mutant Chlamydomonas strain lacking precisely these fibers. The results, which the authors unfortunately only describe qualitatively, are clear: without the fibers the flagella fail to synchronize. These experiments are promising and unequivocally point to the importance of intracellular mechanical coupling. A few lingering issues, however, remain. One of the most immediate is to validate that a single flagellum responds to the periodically driven flow in a way that is consistent with the two-flagella observations.
Pinpointing the origin of intracellular coupling is a remarkable success, but at the same time it opens many new questions: how exactly do the fibers induce in-phase synchronization? Can the cell use the calcium sensitivity of the contractile fibers to modulate the coupling and hence the level, and perhaps the type, of synchrony? And how about the size of the flagella? Previous work found that Chlamydomonas flagella become more synchronized as they grow longer [8]. Could this observation be explained, for example, by assuming that longer flagella cause more stress on the connecting fiber[8]?
Answering these questions will most likely require a detailed quantitative characterization of flagellar coordination across microorganisms with interflagellar connections of different types, something which is already being explored [9]. This is certainly only the beginning of a new and exciting chapter in our understanding of the role played by mechanical forces within the cell.
This research is published in Physical Review Letters.
References
- C. Ainsworth, “Tails of the Unexpected,” Nature 448, 638 (2007).
- E. W. Knight-Jones, “Relations between Metachronism and the Direction of Ciliary Beat in Metazoa,” Q. J. Microsc. Sci. 95, 503 (1954).
- D. R Brumley, K. Y Wan, M. Polin, and R. E Goldstein, “Flagellar Synchronization through Direct Hydrodynamic,” eLife 3, e02750 (2014).
- G. Quaranta, M. Aubin-Tam, and D. Tam, “Hydrodynamics versus Intracellular Coupling in the Synchronization of Eukaryotic Flagella,” Phys. Rev. Lett 115, 238101 (2015).
- J. Elgeti and G. Gompper, “Emergence of Metachronal Waves in Cilia Arrays,” Proc. Natl. Acad. Sci. U. S. A. 110, 4470 (2013).
- F. Dörfler and F. Bullo, “Synchronization in Complex Networks of Phase Oscillators: A Survey,” Automatica 50, 1539 (2014).
- R. E. Goldstein, M. Polin, and I. Tuval, “Noise and Synchronization in Pairs of Beating Eukaryotic Flagella,” Phys. Rev. Lett. 103, 168103 (2009).
- R. E. Goldstein, M. Polin, and I. Tuval, “Emergence of Synchronized Beating during the Regrowth of Eukaryotic Flagella,” Phys. Rev. Lett. 107, 148103 (2011).
- K. Y. Wan and R. E. Goldstein, “Coordinated Beating of Algal Flagella is Mediated by Basal Coupling,” arXiv:1510.03272.