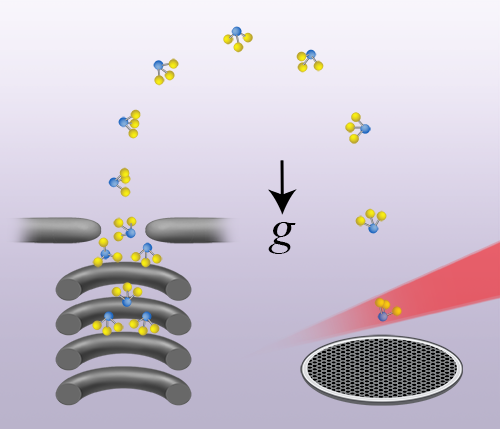
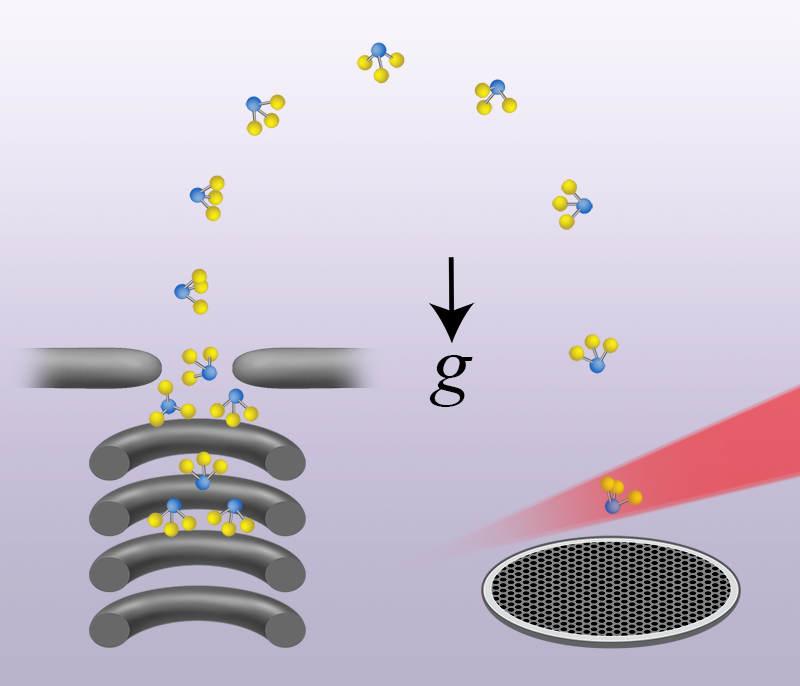
What Goes Up Must Come Down
Serious scientific interest in fountains of matter, in which atoms or molecules are launched upward and allowed to freely fall under the influence of gravity, can be traced back to the 1950s, when Jerrold Zacharias attempted to implement such a device using slow atoms from an atomic beam. Unfortunately, his endeavor was ultimately unsuccessful, being foiled by intrabeam particle collisions, and was relegated to a footnote in scientific history [1]. It wasn’t until the late 1980s, after the techniques of atomic laser cooling became available, that the first atomic fountain was reported [2]. Currently, fountains of ultracold (microkelvin) atoms form the basis of precise measuring devices such as atomic clocks, which realize unparalleled timekeeping accuracy and underpin some of our most advanced modern technologies. Now, for the first time, researchers have demonstrated a fountain based on molecules rather than atoms [3]. Created by Hendrick Bethlem and colleagues from Vrije Universiteit Amsterdam, this new fountain could offer unique access to deviations from the standard model of particle physics and to the conditions present in the early Universe.
A new frontier in experimental physics is currently opening, in which exotic physics is probed not only in large high-energy particle colliders but also in small-scale lab experiments that use molecules at the lowest achievable temperatures. Their rich internal energy structure makes certain molecules exceedingly sensitive to small forces, such as those that would arise from new physics. For example, the current search for a permanent electric dipole moment of the electron using cold molecules [4, 5] tests theories that extend beyond the standard model by probing forces thought to be responsible for the unexplained imbalance between matter and antimatter that formed in the early Universe. Tabletop experiments that use molecules are commonly carried out using molecular beams that are cold, with a narrow speed distribution in the beams’ reference frame, but have a high mean velocity in the reference frame of the lab. This velocity limits the interrogation time—the time during which the particles are observed—and therefore also limits the precision of spectroscopic or interferometric measurements that would detect small forces. Simply making the molecular-beam apparatus longer can increase the interrogation time, but this approach yields diminished returns as the molecules spread out during propagation. Decreasing the temperature of the molecules reduces the spreading and increases the potential interrogation time, and thus a common goal is to make the molecular beam both colder and slower.
A molecular fountain represents the ultimate version of an exceedingly cold and slow molecular beam. In such a device, molecules are brought to rest, trapped, cooled to ultracold temperatures, launched, and measured during freefall. To date, trapped molecules have been too hot to make this approach feasible; the molecules spread out too much during the fountain trajectory. In atomic fountains, ultralow temperatures are achieved using laser cooling. Unfortunately, their complex internal energy structure makes molecules much more difficult to control in this way and, although there has been great recent progress in this direction [6], alternative cooling methods must typically be employed for most molecular species.
In their study, Bethlem and colleagues demonstrate unprecedented control of gas-phase neutral polar molecules, in this case ammonia, by exploiting only the force exerted on the molecules by a (time-dependent) inhomogeneous electric field generated by applying voltages to in-vacuum electrodes. The experiment, illustrated in Fig. 1, begins by bringing a fast molecular beam to rest with a novel hybrid deceleration scheme, which uses both traditional and traveling-wave Stark deceleration techniques. The former method employs rapidly switched high voltages to remove energy from the fast beam and slow it down, but performs poorly at low beam speeds, at which molecules can be captured in a trap. The latter method uses smoothly varied high voltages to create a continuously decelerating three-dimensional potential well to slow the beam. This method works well at low beam speeds and can form a trap in the lab reference frame by bringing the potential well to rest. Once the molecules are trapped, their temperature is lowered by smoothly reducing the trap strength, which narrows the velocity distribution as the molecules expand to fill the larger trap volume. The molecules are then launched vertically upwards and forced to perform a dizzying sequence of acrobatics in both position and velocity space, akin to an Olympic gymnast executing a floor exercise. Next they are allowed to freely fall in a vertical fountain, propagating without any significant forces on them apart from gravity. The position and velocity acrobatics are meticulously designed such that molecules in the fountain have an extremely narrow velocity distribution, and therefore an equivalent ultracold temperature, at the top of the fountain trajectory and exhibit a three-dimensional focus when they return to the launch point. Here they are ionized using a laser beam and detected, being differentiated from rogue molecules present in the background gas by a velocity-sensitive detection technique.
The extreme level of control over the molecules that the researchers attained is an impressive achievement that significantly advances the state of the art in molecule manipulation. While the work is a natural extension of established methods, the results are striking: The fountain increases the possible interrogation time to hundreds of milliseconds, which is a factor of a few hundred larger than that available in a typical molecular beam and should give a correspondingly large improvement in measurement precision in future experiments.
Currently, the experiment produces only a single detected molecule every five repetitions of the launching procedure, resulting in less than one molecule detection per second. Improving this yield will be particularly important to reduce statistical uncertainties in the proposed precision measurements. It appears that straightforward improvements to the setup such as the vacuum pressure, the final trap temperature, and the deceleration efficiency will yield more molecules per shot. Beyond signal improvements, next steps include precision spectroscopy of ammonia to constrain the time evolution of the proton-to-electron mass ratio and thus reveal if the fundamental “constants” of nature have changed over cosmological time scales [7]. Applying the same methods to other species will facilitate investigations into many areas of fundamental physics, from studying parity violation in nuclei [8] or chiral molecules [9], to the search for a permanent electric dipole moment of the electron [10]. Such experiments represent the first steps in the new frontier of using precision measurements of ultracold molecules to improve our fundamental understanding of the Universe and access new regimes in which exciting new physics is waiting to be found.
This research is published in Physical Review Letters.
References
- N. F. Ramsey, Molecular Beams (Oxford University Press, Oxford, 1985)[Amazon][WorldCat].
- M. A. Kasevich, E. Riis, S. Chu, and R. G. DeVoe, “rf Spectroscopy in an Atomic Fountain,” Phys. Rev. Lett. 63, 612 (1989).
- C. Cheng, A. P. P. van der Poel, P. Jansen, M. Quintero-Pérez, T. E. Wall, W. Ubachs, and H. L. Bethlem, “Molecular Fountain,” Phys. Rev. Lett. 117, 253201 (2016).
- J. J. Hudson, D. M. Kara, I. J. Smallman, B. E. Sauer, M. R. Tarbutt, and E. A. Hinds, “Improved Measurement of the Shape of the Electron,” Nature 473, 493 (2011).
- J. Baron et al. (The ACME Collaboration), “Order of Magnitude Smaller Limit on the Electric Dipole Moment of the Electron,” Science 343, 269 (2013).
- J. F. Barry, D. J. McCarron, E. B. Norrgard, M. H. Steinecker, and D. DeMille, “Magneto-Optical Trapping of a Diatomic Molecule,” Nature 512, 286 (2014).
- P. Jansen, H. L. Bethlem, and W. Ubachs, “Perspective: Tipping the Scales: Search for Drifting Constants from Molecular Spectra,” J. Chem. Phys. 140, 010901 (2014).
- D. DeMille, S. B. Cahn, D. Murphree, D. A. Rahmlow, and M. G. Kozlov, “Using Molecules to Measure Nuclear Spin-Dependent Parity Violation,” Phys. Rev. Lett. 100, 023003 (2008).
- M. Ziskind, C. Daussy, T. Marrel, and Ch. Chardonnet, “Improved Sensitivity in the Search for a Parity-Violating Energy Difference in the Vibrational Spectrum of the Enantiomers of CHFClBr,” Eur. Phys. J. D 20, 219 (2002).
- M. R. Tarbutt, B. E. Sauer, J. J. Hudson, and E. A. Hinds, “Design for a Fountain of YbF Molecules to Measure the Electron’s Electric Dipole Moment,” New J. Phys. 15, 053034 (2013).