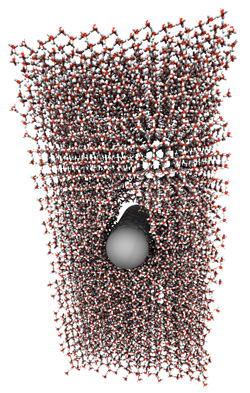
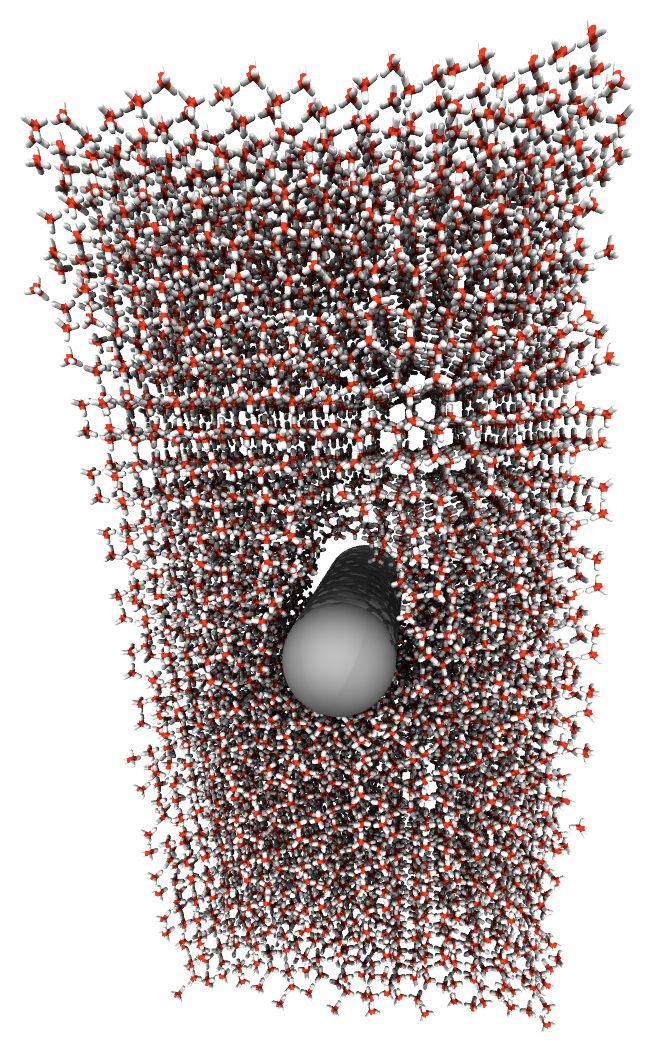
Cutting Ice with a Nanowire
In a classic experiment, a wire weighted at each end gradually slices down through a block of ice because of frozen water’s unusual property of melting under pressure. In the 20 August Physical Review Letters, researchers describe simulating this experiment at the molecular level on a computer. They discovered that it’s harder to push a hydrophobic (water-repelling) wire through ice than a hydrophilic (water-attracting) one. The analysis reveals new details of the interaction of water and ice with a hydrophobic object.
In the mid-19th century, Michael Faraday, J. Willard Gibbs, and James Thomson were among the first to study the wire experiment, which demonstrates liquid water’s peculiar feature of being denser than ice. As the wire slices into the ice, the water above the wire refreezes. But until now, the experiment has only been studied at the macroscopic scale. The process changes at smaller scales, where water is no longer a continuous fluid, but a network of individual molecules. Zooming in might reveal unknown behaviors and would allow researchers to focus on an ideal system devoid of macroscopic complications, such as friction due to defects and impurities, says Teemu Hynninen of Tampere University of Technology in Finland.
Hynninen and his colleagues simulated the experiment with a model of between 1000 and 10,000 frozen water molecules and hydrophobic and hydrophilic wires with radii of 7.5, 9.0, and 10.5 angstroms. The wires started at rest inside the ice and were then pushed with varying amounts of force. The team then measured the velocities of the molecules and the wire. Conventional thinking suggests that a hydrophobic wire, whose surface interacts less with water molecules and should therefore have less friction, would move more easily than a hydrophilic wire. But the researchers discovered the opposite.
They found that in the hydrophilic case, a thin layer of newly melted ice surrounds the moving wire and flows around it. But for a hydrophobic wire, the water accumulates underneath, rather than flowing around. Meanwhile, the wire still pushes through the liquid–albeit at a slower pace, because of the watery road block–and leaves behind a small cavity above it. Eventually, after enough water has built up, the liquid seeps up around the wire and rushes to fill the empty space, allowing the wire to speed up slightly before slowing down as the water layer accumulates again. This process repeats in a cycle, so that the wire alternates between faster and slower motion through the ice.
In either case, the wire requires a minimum amount of force in order to move, so the researchers saw a transition from stationary to moving as they ran simulations with different values for the force. The properties of this transition depended on the type of wire. For the hydrophobic wire, the transition point depended on whether the force was being increased or decreased, an effect called hysteresis. With a gradually increasing force, the wire can’t move until enough liquid water collects below it, but when the force is decreasing, the necessary pool of liquid water persists, allowing the wire to move even with somewhat less force pushing downward. The hydrophilic wire, on the other hand, shows no hysteresis, because it doesn’t produce a reservoir of water. In fact, it’s surrounded by a thin layer of liquid even in its stationary state, so the two states are very similar.
“The fact that it’s easier to cut ice with a hydrophilic wire than a hydrophobic wire is, for me, quite unexpected,” says Riccardo Ferrando of the University of Genoa in Italy. Computer simulations rarely provide clear and surprising predictions like this one, he adds. “The behavior of water and ice is one of the most interesting things someone can study–there are always surprises.”
–Marcus Woo
Marcus Woo is a freelance science writer in the San Francisco Bay Area.