Self-Pinching Smoke Rings
6 December update: See note below.
The mixing of two fluids is a complex process, but it’s even more complex when the two participate in a chemical reaction. Now researchers publishing in the December Physical Review E have shown that a self-sustaining reaction in a fluid-filled tank can generate a series of vortex rings, similar to smoke rings, without any external forcing. Researchers say the effect hints at what might be possible in more exotic situations such as under the sea, deep inside the Earth, or even in stars.
When two different fluids come together under the right conditions, they may produce an instability–a severe deformation of the boundary between the liquids. A classic example is the Raleigh-Taylor instability, in which a more dense fluid plows down into a less dense fluid. Instabilities happen all the time in the atmosphere, the oceans, and inside stars. However, researchers know little about the types of instabilities that form when chemical reactions are taking place at the interface between two fluids.
One example is the so-called iodate-arsenous acid system, where some of the reaction products also serve as catalysts of the same reaction, making it self-sustaining. Once a tiny amount of the catalysts is introduced into a solution containing the reactants, the reaction produces a wave front that leaves in its wake a more buoyant fluid, which grows in volume. In the past, researchers have studied how the interface between these two solutions moves up or down thin capillary tubes or slots, approximately 1 millimeter wide. But Stephen Morris of the University of Toronto and his then PhD student Michael Rogers wanted to give the reaction more room to maneuver. They filled a 9-centimeter-wide, vertical cylinder with a solution of the reactants and introduced a small amount of the catalysts through a capillary tube in the bottom.
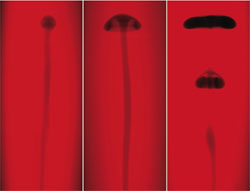
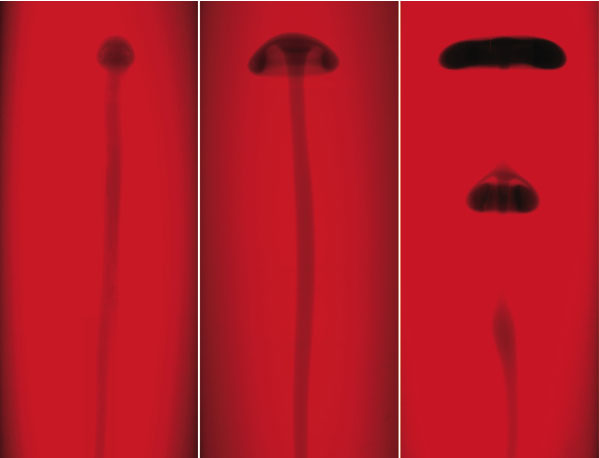
The researchers performed the experiment several times using different amounts of glycerol in the liquid. In all cases, the reaction front formed a thin, vertical tube, or “conduit,” that crept upward at a rate of a few centimeters per minute. When the reactants were dissolved in pure water, the conduit was capped with a small plume head, like an elongated mushroom. But when glycerol was present, the plume head broadened and wrapped under itself, and at concentrations of glycerol between 30 and 40 percent, the plume formed one or more so-called vortex rings (like smoke rings) that detached themselves from the conduit. In a non-chemically reactive system, a physical push would be required to generate a self-pinching plume, and even then it’s tricky to do, Morris says.
The key to the effect is the relative buoyancy of the reacted liquid, which is warmer and less dense than the starting solution. At first, the buoyancy of the conduit feeds the upward motion of the plume head at its tip. But the group’s computer simulations, constructed by the late Abdelfattah Zebib of Rutgers University, indicate that early on in the process, the plume begins to accelerate. “The head pinches off because it accelerates away from the fluid beneath it,” Rogers says, “and it does this because it is constantly generating additional buoyancy by reacting with its surroundings.” The fluid in the conduit also accelerates, which, depending on the concentration of glycerol, leads to a pooling of reacted liquid that forms a new vortex ring below the initial one.
“The authors have taken a system that has been studied for over 20 years and found new phenomena,” says chemist John Pojman of Louisiana State University in Baton Rouge. He says the plumes in the new study are more complex than those produced by simple heating or differences in composition between two fluids. “It shows that totally new dynamics can be expected if you look at classical instabilities but using reactive fluids,” adds physical chemist Anne De Wit of the Free University of Brussels (ULB). She says there are likely to be many examples of such instabilities in ocean currents, flows of molten rock inside the Earth, and plasma flows inside stars. Researchers often don’t account for the effects of chemistry on fluid flows, says De Wit, but these results show that “you cannot ignore them.”
6 December update: Initial observations of the vortex rings were originally published in 2005 [1]. The new paper presents the effects of viscosity and the results of computer simulations, which allowed the authors to fully explain the pinch-off phenomenon and other aspects of the process.
–JR Minkel
JR Minkel is a freelance science writer in New York City.
References
- M. C. Rogers and S. W. Morris, “Buoyant Plumes and Vortex Rings in an Autocatalytic Chemical Reaction,” Phys. Rev. Lett. 95, 024505 (2005)