Tumbling of Molecules Affects Experiments
Researchers can learn a lot about a molecule and its environment by measuring the spectrum of electrons it ejects in response to a blast of radiation. With improved equipment and techniques, experimentalists are getting close to measuring the true, intrinsic shape of the features in such electron spectra. But in the 13 May Physical Review Letters a team shows that the improvements have exposed an effect that has previously been ignored: the rotation of molecules in a gas can significantly affect the spectrum. Researchers call this “rotational Doppler broadening,” although it doesn’t fit the usual definition of the Doppler effect. It could present a new limit to the most precise measurements.
Electrons dislodged by x- rays provide information about solid surfaces or molecules and also about motions within a molecule in response to the loss of an electron. For example, the width of a feature in the spectrum of electron energies can tell you exactly how long the molecule took to rearrange. So accurately determining the width is essential, especially as the latest synchrotrons have improved the precision of these photoelectron measurements.
For a gas, one important effect on the spectrum comes from the molecules’ motion. If a molecule moves toward or away from the x-ray source, the Doppler effect slightly increases or decreases the apparent frequency of the x-ray photon, and thus its energy. Since various molecules have different speeds and directions, this effect spreads a sharp feature in the spectrum into a broader peak. This so-called Doppler broadening is one of the remaining limits to better resolution in x-ray photoelectron spectroscopy. Now T. Darrah Thomas of Oregon State University in Corvallis and an international team of collaborators have shown that the thermal rotation of molecules is just as important.
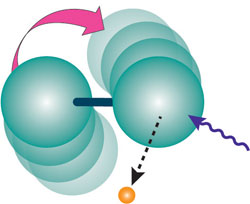
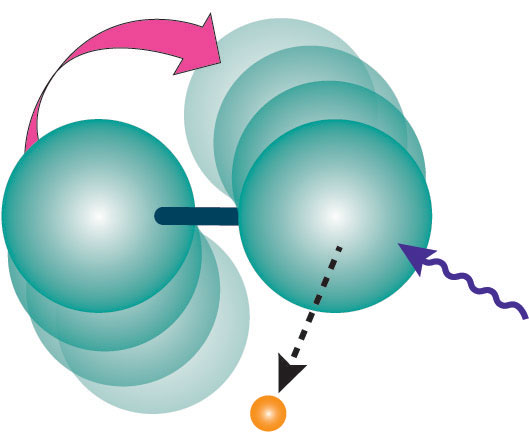
If a dumbbell-shaped molecule tumbles, and one atom emits an electron in the direction it’s moving, the electron will get a boost in energy compared with an electron emitted in the opposite direction. The combination of different rotations and emission directions broadens the peak in the electron spectrum, similar to Doppler broadening. “Rotational Doppler broadening” is the term some spectroscopists have been using for the effect, even though it’s unrelated to any photon frequency shifts. “It had been obvious to me that these effects were there, and it was obvious to me a long time ago what the explanation was,” says Thomas. But it took time to do all the experiments and analysis.
The team shined x- rays of a single energy on nitrogen molecules, and measured how increasingly vigorous rotation of the molecules–from increasing temperature–increased the range of energies of dislodged electrons. In one test, the researchers re-analyzed the temperature-induced broadening seen in earlier experiments from Erwin Poliakoff of Louisiana State University in Baton Rouge and colleagues. In a second test, they took new data to compare the broadening for nitrogen with that for krypton gas, which is made of single atoms, rather than molecules, and should have no rotational effect. The observed energy spread agreed quantitatively with the predictions of the team’s simple, dumbbell-like model. The results also matched a more complete quantum-mechanical calculation published last year [1].
The rotation-induced broadening is similar in magnitude to the Doppler broadening and will limit the ultimate resolution that the experiments can achieve, according to the team. What’s more, because rotational Doppler broadening does not arise from molecular motion relative to the photons, Thomas says, “the techniques people have developed for Doppler-free broadening are not going to work here.”
“Instrumentally, what they’re doing is pretty impressive,” because the observed broadening is such a small fraction of the emitted electron energy, says Poliakoff of the new experiments. He adds that many physicists (but perhaps not chemists) would have expected that “the molecular aspects of the problem would peter out” for such high photon energies. “The fact that it’s something which in retrospect is so obvious, and was so completely elusive to people for decades, points out that it’s actually pretty important.”
–Don Monroe
Don Monroe is a freelance science writer in Murray Hill, New Jersey.
References
- Yu-Ping Sun, Chuan-Kui Wang, and Faris Gel’mukhanov, “Rotational Doppler Effect in X-ray Photoionization,” Phys. Rev. A 82, 052506 (2010)