Cells in a New Light
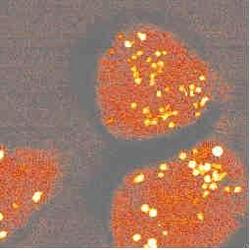
To study cells under a microscope, researchers often mark specific cell structures with stains or fluorescent dyes. Some proteins even light up without the addition of a dye. But not all components of cells can be made to fluoresce by these methods. The 17 May PRL describes a laser-based method for imaging parts of a live cell in three dimensions based on the vibrations within molecules in the cell. As a proof-of-principle, the authors show an image of the mitochondria in a cell, created by aiming lasers at the cell but without disturbing it in any other way. The authors plan to study molecules that can’t be seen using fluorescence or for which artificial dyes would interfere with the observations.
Fluorescence techniques allow biophysicists to make high-resolution three-dimensional images of cells and detect single protein molecules. Andreas Zumbusch, now of the Ludwig Maximilians University in Munich, Germany, Gary Holtom of the Pacific Northwest National Laboratory in Richland, WA, and Sunney Xie, now of Harvard University, wanted to image cellular components that don’t tolerate dyes or fluoresce on their own. The technique they developed, called coherent anti-Stokes Raman scattering (CARS) microscopy, was introduced in the early 1980s in a less sophisticated form by a group at the Naval Research Laboratory in Washington, DC, but they were not able make high quality three-dimensional images.
The idea of CARS microscopy is to detect molecules whose atoms vibrate at a specific frequency, such as the stretching frequency characteristic of carbon-hydrogen bonds, which are common to the long hydrocarbon chains found in cell membranes. To image these molecules in a cell, the team sent in two tightly focused laser beams of different wavelengths: a “pump” beam at about 855 nm and a “Stokes” beam at about 1.2 µm. When the beams were tuned so that the difference in energy between pump and Stokes photons was exactly the energy of the C-H vibration, a strong light beam (called “anti-Stokes”) was produced by the interaction of the photons with the hydrocarbons. The process requires two pump photons and one Stokes photon for every Anti-Stokes photon it produces, so only a small volume of the cell received sufficient intensity and overlap of the lasers to cause a response. By moving the beam-overlap region relative to the sample and detecting the intensity of anti-Stokes light from each position, the team mapped the concentration of hydrocarbon chains throughout a cell. The image shows the mitochondria most clearly because they contain the highest concentration of hydrocarbons.
Although CARS microscopy does not require dyes, the current version cannot work fast enough to follow fast changes in cells, as fluorescence microscopy can, and fluorescence is also more sensitive. But the CARS process is much less damaging to the molecule being detected, and almost all of the emitted photons can be collected because they emerge in a coherent beam, rather than in all directions.
“Reviving this technique is a nice idea and may provide some useful information,” says Wat Webb of Cornell University. But he’s skeptical that CARS can be useful in biology given the low speed and sensitivity presented in the paper. Holtom says improvements are already planned to address those concerns.


