Membrane Inclusions and Protrusions
Nanotechnology may one day play a starring role in drug delivery, but researchers need to understand more about the mechanical forces between nanoparticles and biological membranes. Previous studies showed how a single nanoparticle can distort the shape of a vesicle membrane. Now, based on new computer simulations, two groups report in Physical Review Letters that unexpected tubelike structures can form when multiple nanoparticles are involved. Studying the structures could point researchers to new ways of encapsulating nanoparticles, or help them understand how viruses permeate and infect cells.
A chemically “sticky” nanoparticle can wrap itself partially or entirely in the membrane of a much larger vesicle or cell, providing a route of entry into a cell, or a stable position in tissue. But this wrapping creates a bend in the membrane, which costs energy.
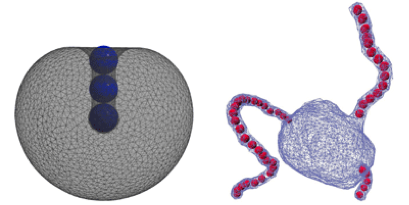
Amir Bahrami and colleagues at the Max Planck Institute of Colloids and Interfaces in Potsdam, Germany, wanted to understand how the balance between adhesion and bending was affected by the presence of multiple particles, which can interact with each other through the elastic forces in the membrane. They assumed that two or three nanoparticles adhered to the outer surface of an elastic spherical membrane and calculated the vesicle shapes and nanoparticle positions that minimized the total energy. Several scenarios emerged where the particles formed dimer or trimer bound states inside of a tubule of the vesicle. Since the structures depend on the area-to-volume ratio of the vesicles, the authors suggest osmotic pressure, which affects this ratio, could be used to reversibly encapsulate the particles.
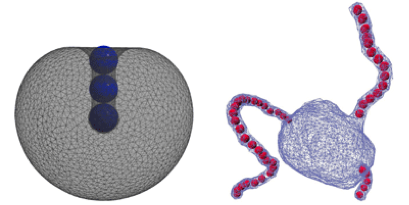
Independently, Anđela Šarić and Angelo Cacciuto at Columbia University, New York, considered nanoparticles that were inside the vesicle, rather than outside. They showed that the self-assembly of nanoparticles into strings induces the spontaneous formation of tubular structures that protrude from the membrane. Compared to the “buds” that viruses form on cell membranes, the development of these tubular structures requires less adhesion energy, pointing to a potentially unexplored route of viral infection. – Jessica Thomas