Hydrogen Clusters Go Negative
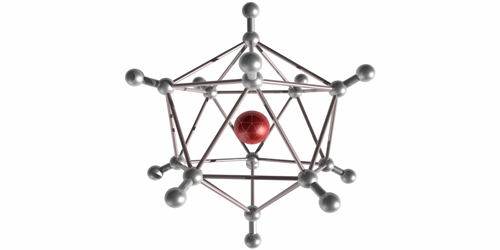
At very low temperatures, hydrogen (H) can form ionized clusters containing as many as a hundred atoms. Previously only observed with positive charge, researchers have now detected negatively charged clusters. The number of hydrogen atoms in these clusters is always odd, but certain numbers of atoms appear more often than others. The results suggest that the clusters are based on a solid-like structure in which a single ion is surrounded by an ordered arrangement of molecules.
Theorists long ago predicted that hydrogen clusters with large atom numbers may form in outer space, and lab experiments confirmed the existence of positively charged clusters 40 years ago. Negatively charged clusters (or anions) have proved more elusive. Until now, the largest hydrogenic anion was .
In their experiment, Michael Renzler from the University of Innsbruck in Austria and his colleagues injected molecules into cold liquid-helium droplets, where the molecules form neutral clusters. When the team exposed the droplets to an electron beam, the clusters ionized and escaped into the surrounding vacuum. The clusters were stable for at least several microseconds—long enough for them to reach a mass spectrometer that recorded a range of odd atom numbers from to . The odd values implied that the clusters were a combination of several molecules and a single ion core, held together through an induced dipole attraction. The highest observed abundances were for , , and . These “magic numbers” correspond to symmetrical solid-like arrangements of molecules, such as a 20-sided icosahedron. Future studies may explore the chemistry of these large anions and possible quantum effects in the vibrational excitations of the crystalline structures.
This research is published in Physical Review Letters.
–Michael Schirber
Michael Schirber is a Corresponding Editor for Physics based in Lyon, France.