Silica’s High-Pressure Phase
Silicon dioxide ( ), commonly known as silica, is a model system for studying how the structure of an amorphous material changes when it is subjected to high pressures. Despite decades of experiments, however, researchers haven’t yet pinned down the various high-pressure phases to which transitions when it is rapidly compressed. In new shock-compression experiments at an x-ray synchrotron, Sally June Tracy at Princeton University, New Jersey, and colleagues observed that transforms from an amorphous material to a crystal at a pressure of 36 GPa. They also determined the structure of the crystalline phase, which they found forms within less than one microsecond. The results could improve models of meteorite impacts and explosions in the Earth’s crust, where is the most abundant oxide.
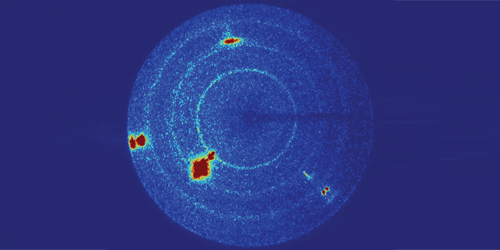
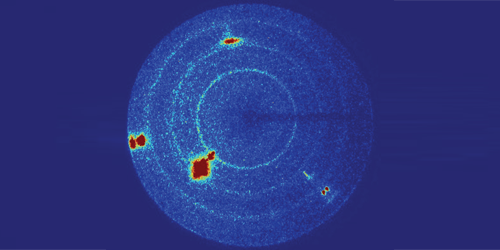
Tracy and colleagues fired plastic “bullets” at a slab of glassy and monitored changes in the material’s structure using x-ray diffraction. By varying the bullets’ velocity, the team controlled the pressure of the shock wave created in the sample by the bullets’ impact. For pressures lower than 34 GPa, no diffraction peaks appeared in the data, indicating that the material remained a glass. For pressures of 36 GPa and higher, sharp peaks developed, demonstrating that the material had transformed into a crystal. Comparing the experimental data with predictions from simulations, the team determined that the crystalline phase had a stishovite structure—a tetragonal form of commonly found deep in Earth’s mantle.
serves as an archetype for other silicates found in Earth’s layers, such as feldspars and olivine. So these results could help researchers understand the shock-compression behavior of myriad rocks and minerals.
This research is published in Physical Review Letters.
–Katherine Wright
Katherine Wright is a Contributing Editor for Physics.