Reining in Diffusion in Dense Liquids
Two quantities largely determine liquid dynamics: viscosity and the diffusion coefficient. Viscosity is a macroscopic measure of the resistance of the fluid against shear deformation, and the diffusion coefficient measures the long-range atomic motion. At temperatures far from the freezing point, they are connected by the Stokes-Einstein relation (SE), which holds if the fluid is sufficiently above the liquidus temperature. However, a variety of studies have found that the SE relation is not adequate to describe liquids closer to the glass. One important unresolved question has been how far one can cool down before the SE relation breaks down. Now, writing in Physical Review Letters, Jürgen Brillo and coauthors from Deutsches Zentrum für Luft- und Raumfahrt, Germany, show that the Stokes-Einstein relation not only breaks down at much higher temperatures than anticipated, but also gives the wrong temperature dependence [1]. These results suggest that great caution is needed when the SE relation is used to express diffusivity in terms of viscosity, or vice versa, and that a new description needs to account for the correlations between the motion of single atoms.
Shear viscosity, usually simply called viscosity, is a macroscopic measure of the resistance of a fluid against deformation by a shear-keeping laminar flow. According to Stokes law, when a sphere of radius is dragged with velocity through a liquid with viscosity , a force is needed. Using linear response theory, the Stokes-Einstein relation can be derived [2]. In equilibrium, the concentration of such spheres is given by Boltzmann’s formula . Taking , the concentration gradient is given by . Equilibrium requires that the current of particles due to the external force is canceled by the diffusive current . Solving for , the Stokes-Einstein relation [3] between viscosity and diffusion coefficient results:
It is valid for the diffusion of uncorrelated macroscopic spheres in a liquid. Treating the motions of the solvent atoms as uncorrelated, the SE relation is applied to the diffusion of single atoms or molecules. At sufficiently high temperatures, the SE relation holds well and many textbooks give it as a recipe to derive from and vice versa. While some experimental variability has been observed, discrepancies of up to can be absorbed in an effective hydrodynamic radius, , and a reduction of the factor 6 in the denominator, which is equivalent to assuming there is some surface slip [4].
However, when the liquid is cooled, the atomic motion gets more and more correlated and the SE relation will eventually break down. In the absence of accurate measurements of and , it was assumed that the relation will hold above the critical temperature , a critical temperature obtained from mode coupling theory for undercooled liquids [5]. This theory is designed to describe the decay of correlation functions on time scales much larger than the picosecond scale of atomic collisions or vibrations. This slow decay is given by a memory function, which is written in terms of the static structure factor. Since the structure factor is an average over all atoms, it is a mean field theory. Nevertheless, the theory is able to describe or predict a large number of glassy properties of undercooled liquids and can be extended to more complex systems. Cooling to , which is typically about higher than the glass transition temperature, fluidlike motion is thought to freeze out, following scaling laws , with a material dependent exponent. In real systems, thermally activated hopping becomes the dominant mechanism for diffusion.
In contrast to the conventional wisdom, computer simulations have pointed to a breakdown of the SE relation at much higher temperatures, examples of which are given in the article by Brillo et al. [1]. Experimental validations of the SE relations, so far, have suffered from using different samples that were held under different conditions for the measurements of and . A particular problem has been the influence of the containment. In the electrostatic levitation technique, a droplet of liquid metal is held in position by a suitable static field, thus eliminating the container problem. Using this technique, Brillo et al. [1] have measured both and in liquid to a high precision over a temperature range of . As expected, they find that the SE relation somewhat underestimates the diffusivity. More importantly, and representing a real challenge to theory and experiment, they find
in striking contrast to , which is what is predicted by the SE relation. The relation found by the team is seen in mode coupling theory near , but this does not explain why still holds above the liquidus temperature . Therefore, a basic assumption in the derivation of the SE relation at any temperature is shown to be violated, and correlations between the atomic motions persist even at very high temperatures.
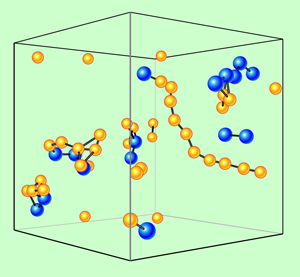
Deviations from the SE relation have also been found in previous studies. In diffusion experiments and in simulations of metallic glasses [6], results point to collective processes both in the glass and the melt. If an atom diffuses through a rigid lattice, its diffusion coefficient is inversely proportional to the square root of its mass, . This holds approximately for the diffusion via vacancies in crystals. In contrast, by changing the mass of a tracer atom (i.e., by using a different isotope) it was found that in both glasses and supercooled melts the diffusion coefficient of the atom is nearly independent of its mass. This “vanishing isotope effect” indicates a collective motion of ten or more atoms [7] in the melt. Computer simulations show cluster and string structures moving through the melt, as seen in Fig. 1. A recent simulation of diffusion in showed that up to above . At higher temperatures, diffusion followed the SE behavior [8]. This switchover on cooling is related to the onset of a regime of collective motion of strings or chains of atoms. Instead of individual atoms moving through the melt, as is assumed in Stoke’s law and the SE relation, groups of atoms move together. This is accompanied by large variations of the mobility of different atoms at any given time. It is similar to the behavior of a dense crowd of people, where the strings resemble small chains of people threading their way through the slowly moving mass. These “annoying” people get ahead much faster and fill any small gap opening.
The collectivity of motion invalidates the assumptions made in the derivation of the SE relation, but a theory that explains the observed at high temperatures is outstanding. The question of how chain motion interacts to create viscous flow is unsolved. The results of Brillo et al. show we have much to understand about how the motion of atoms in dense liquids affects their macroscopic properties.
References
- J. Brillo, A. I. Pommrich, and A. Meyer, Phys. Rev. Lett. 107, 165902 (2011)
- L. D. Landau and E. M. Lifshitz, Fluid Mechanics (Pergamon Press, Oxford, 1987)[Amazon][WorldCat]
- A. Einstein, Ann. Phys. 17, 549 (1905)
- U. Balucani and M. Zoppi, Dynamics of the Liquid State (Clarendon Press, Oxford, 1994)[Amazon][WorldCat]
- W. Götze and L. Sjögren, Rep. Prog. Phys. 55, 241 (1992)
- F. Faupel et al., Rev. Mod. Phys. 75, 237 (2003)
- H. Ehmler, A. Heesemann, K. Rätzke, and F. Faupel, Phys. Rev. Lett. 80, 4919 (1998)
- X. J. Han and H. R. Schober, Phys. Rev. B 83, 224201 (2011)
- H. R. Schober, C. Gaukel, and C. Oligschleger, Prog. Theor. Phys. Suppl. 126, 67 (1997)