How to Turn an Embryo Inside Out
We all start life as a small ball of cells. These cells proliferate and organize into tissues, which in turn grow and fold into the complex form that defines a human being. The process that leads from a formless ball to a functional organism—known as morphogenesis—is an active area of research in biology. For physicists, it presents an exciting challenge because a growing organism can be thought of as a type of “active” matter that is capable of reshaping itself [1,2]. Physics models are therefore increasingly being used to investigate morphogenetic events in plants [3] and animals [4].
Now, researchers at the University of Cambridge in the UK have compared images of a growing algal embryo with a physics-based morphogenesis model, providing one of the few experimental tests of such models [5]. Stephanie Höhn and her colleagues imaged embryos of the algal species Volvox globator as they underwent invagination—a fundamental event in morphogenesis in which a sheet of cells folds inside, triggering the formation of an internal cavity. The researchers modeled the organism as a spherical elastic shell that actively bends and contracts at certain segments, and they found the necessary balance between these motions for inversion to occur.
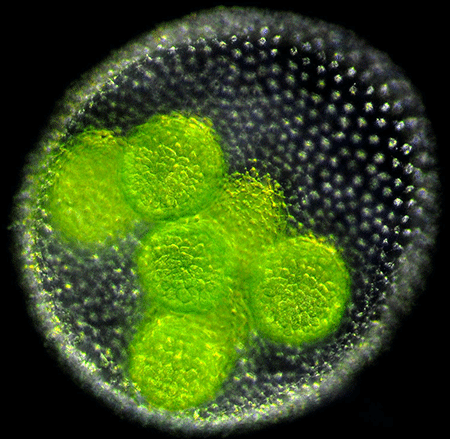
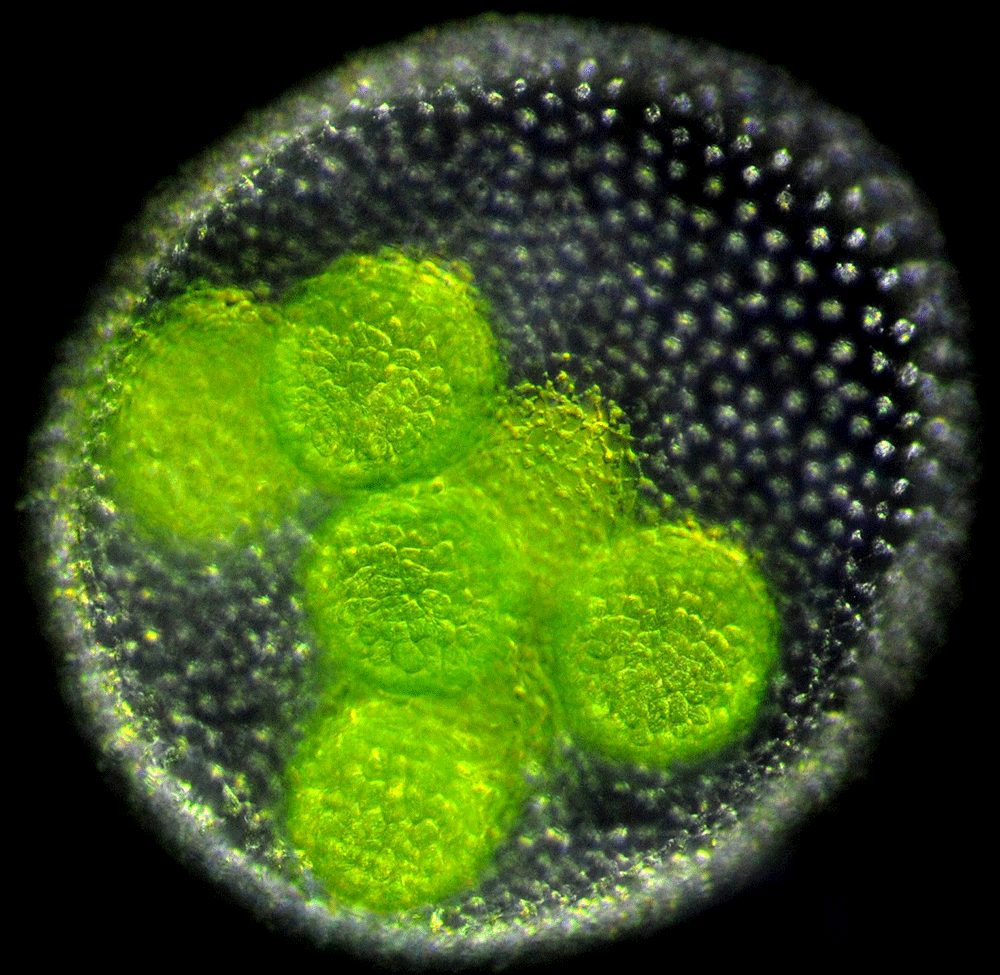
Volvox are freshwater algae generally found in ponds [6]. An adult Volvox consists of a single layer of a few thousand cells embedded within a gelatinous extracellular matrix that encloses a spherical cavity. Each cell has two flagella that face outwards and beat in the water, allowing the spheroid to swim towards light and nutrients. Embryo Volvox develop within a parent spheroid (Fig. 1). And, unlike adults, their flagella are oriented inwards. To form an adult, the embryonic sheet of cells must therefore turn itself inside out.
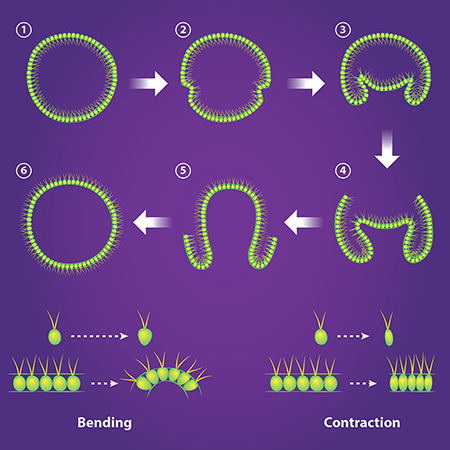
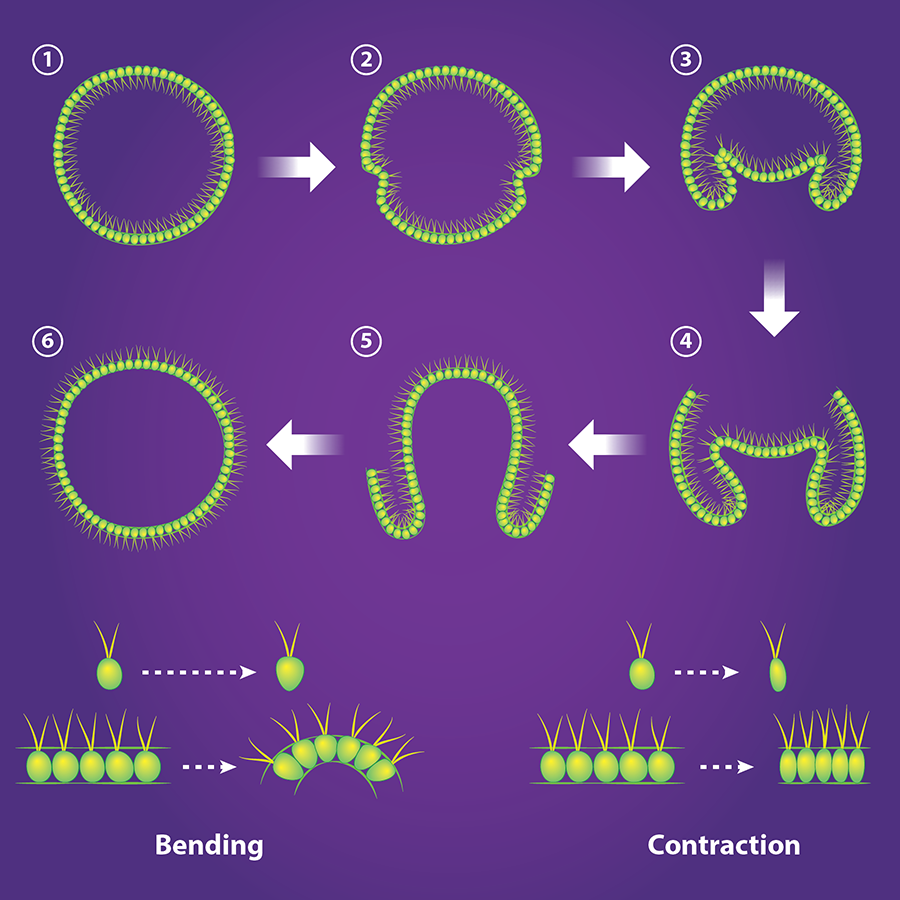
This stunning process of inversion varies from species to species [7]. In V. globator, one half of the spherical sheet of cells shrinks in radius and invaginates, initiating inversion (Fig. 2, top). In 2011, Höhn and Armin Hallmann [8] obtained, at different stages of inversion, the cross-sectional shape of the V. globator embryos. They also imaged the shape of the cells in the spherical sheet, which allowed them to relate the macroscopic shape of the embryo to the microscopic behavior of the cells. In their new work [5], Höhn et al. observed the live inverting embryos in three dimensions and quantified their changing shapes in real time. They also constructed and validated a continuous mechanical model that provides a tissue-based view of how inversion occurs.
Embryo Volvox are small (70–90 micrometers) and their growth is highly sensitive to external probes, such as intense light or an electron beam. Both factors make imaging the organism a challenge. To reduce the organism’s exposure to intense light, Höhn and her collaborators used a high-resolution optical technique (selective plane illumination microscopy [9]) that illuminates and collects light from just a slice of the Volvox embryo. The images were then pieced together to form a 3D image. Höhn et al. imaged the embryos at regular time intervals throughout inversion, at each time step tracing the contour of the cell sheet. From the contours, the team could measure the depth of invagination, the area of the cell sheet, and the curvature along the sheet.
With this quantitative data in hand, the researchers tried to find the simplest mechanical model that reproduced the shape changes in the embryo as it began the invagination process. As a starting point, they modeled the embryo as a thin elastic shell that was initially spherical. The researchers then derived an equation that predicts the shape of the shell when a segment bends or contracts. They first tested the idea that the spherical shell only bends at its equator. This process is activated by the cells—which start off roughly ellipsoidal—becoming more cone-like, with their wide ends facing outwards (Fig. 2, bottom). They then solved their equation numerically to predict embryo shapes. Active bending alone, they found, doesn’t reproduce the shape of V. globator as it begins inversion. (It does, however, reproduce the shape of another species, Volvox aureus.) Höhn et al. next assumed that the shell can also contract, which can be activated by the cells elongating (Fig. 2, bottom). By balancing bending and contraction, they were able to reproduce—quantitatively—the embryo geometry during the first stages of inversion.
Recent studies have, like Höhn et al., shown that contraction is the most efficient way to change the geometry of a thin sheet of tissue [10] (see 3 October 2008 Focus). The work of Höhn et al. [5] also brings a new ingredient—active matter—to the physics of thin shells, a surprisingly difficult system to describe theoretically [11]. For example, a ping-pong ball under a heavy weight can’t make a smooth transition from round to flat; instead, the force induces dimples and folds—the only way the ball can flatten is if regions of the shell contract.
The success of the work by Höhn et al. raises a number of exciting questions. For instance, is the hypothesis of active bending required to retrieve observations? An alternative hypothesis, for example, is that the conical shape of cells is merely a passive consequence of active stretching in other regions of the embryo. Another open question is whether the model is general enough to capture later stages of inversion, when the cell sheet reconnects. These questions could likely be addressed with more theoretical work. Nevertheless, the main ingredients in Höhn et al.’s model—bending and contraction—are generic. By varying the weight of these two ingredients, the model should, in principle, be applicable to other organisms with invaginating thin tissues.
This research is published in Physical Review Letters.
References
- M. C. Marchetti, J. F. Joanny, S. Ramaswamy, T. B. Liverpool, J. Prost, M. Rao, and R. Simha, “Hydrodynamics of Soft Active Matter,” Rev. Mod. Phys. 85, 1143 (2013)
- J. Prost, F. Jülicher, and J-F. Joanny, “Active gel physics,” Nature Physics 11, 111 (2015)
- O. Ali, V. Mirabet, C. Godin, and J. Traas, “Physical Models of Plant Development,” Annu. Rev. Cell Dev. Biol. 30, 59 (2014)
- M. Rauzi, A. H. Brezavšček, P. Ziherl, and M. Leptin, “Physical Models of Mesoderm Invagination in Drosophila Embryo,” Biophys. J. 105, 3 (2013)
- Stephanie Höhn, Aurelia R. Honerkamp-Smith, Pierre A. Haas, Philipp Khuc Trong, and Raymond E. Goldstein, “Dynamics of a Volvox Embryo Turning Itself Inside Out,” Phys. Rev. Lett. 114, 178101 (2015)
- J. E. Graham, L. W. Wilcox, and L. E. Graham, Algae (Benjamin Cummings, San Francisco, 2009)[Amazon][WorldCat]
- A. Hallmann, “Morphogenesis in the Family Volvocaceae: Different Tactics for Turning an Embryo Right-side Out,” Protist 157, 445 (2006)
- S. Höhn and A. Hallmann, “There is More Than One Way to Turn a Spherical Cellular Monolayer Inside Out: Type B Embryo Inversion in Volvox globator,” BMC Biology 9, 89 (2011)
- J. Huisken, J. Swoger, F. Del Bene, J. Wittbrodt, and E. H. K. Stelzer, “Optical Sectioning Deep Inside Live Embryos by Selective Plane Illumination Microscopy,” Science 305, 1007 (2004)
- J. Dervaux and M. Ben Amar, “Morphogenesis of Growing Soft Tissues,” Phys. Rev. Lett. 101, 068101 (2008)
- L. D. Landau and E. M. Lifshitz, Theory of Elasticity (Pergamon, Oxford, 1986)[Amazon][WorldCat]